Women need to be at the heart of clinical trials
London School of Hygiene & Tropical Medicine https://lshtm.ac.uk/themes/custom/lshtm/images/lshtm-logo-black.png Thursday 16 May 2024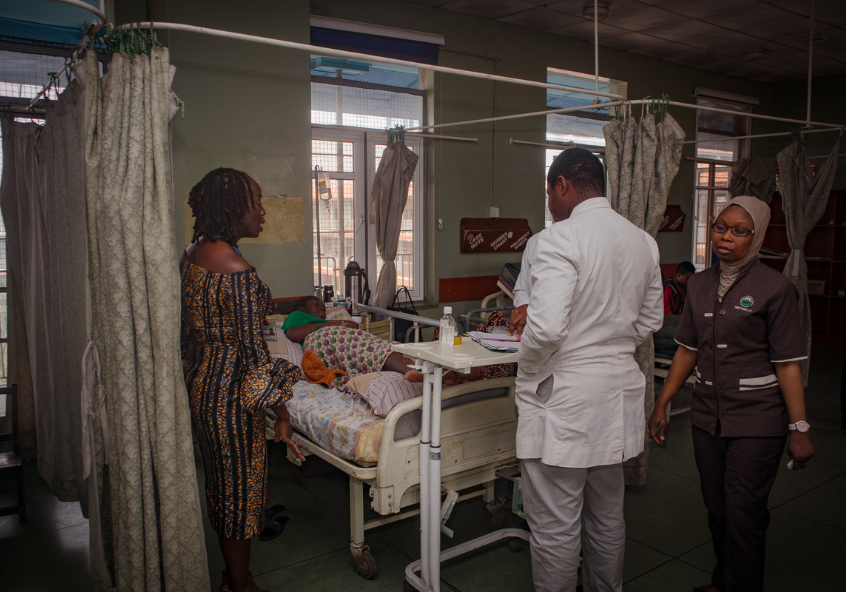
Professor Nike Bello, head of the WOMAN-2 Trial in Nigeria, at the maternity ward of University College Hospital, Ibadan.
What’s the issue?
Across many types of trials, the recruitment and retention of women is a challenge. In cardiovascular trials, approximately a third of participants are women.
Why is there gender inequity in clinical trials?
It’s often extremely challenging to recruit people to take part in clinical trials under any circumstances, so the focus has been on recruiting sufficient numbers rather than the ratio of men and women.
Research has shown that women are often excluded from trials due to their age and health status. Follow-up visits can also be a barrier for women with children or caring responsibilities. In some cultures partners and family members expect to be involved in decision making, which is not usually addressed well in trials.
There is also a perception that women are more difficult to enrol into trials than men. In a resource-strapped system, research nurses may understandably feel forced to choose the option that takes less resource. True or not, this could also be because trials have been designed without women’s needs in mind.
What are the challenges for cardiovascular trials in particular?
Heart disease is largely considered a male issue, despite cardiovascular disease being the number one cause of death for women globally.
Cardiovascular trials are a male-dominated field at all levels – funders, researchers, consultants and patients.
The biggest challenge is underdiagnosis of heart problems in women. Women may describe their symptoms differently; they are more likely than men to have a heart attack with no severe blockage in an artery; medical professionals may prioritise other aspects of their symptoms; and it often takes longer for women to be diagnosed.
When it comes to treatment, fewer women receive interventions such as heart bypass surgery or non-surgical procedures, which trials are often designed around. Because there is an idea that it’s challenging to recruit women to cardiovascular trials, there is sometimes a reluctance to fund research in women. This clearly needs to change in order to address this issue.
Why does it matter if not many women take part in trials?
If the evidence is based predominantly on male participants, the results may not be applicable to women. This inequity means there is limited evidence for the best way to treat women, which could potentially lead to a lower quality of life, higher mortality, more hospitalisations and co-morbidities.
Women have additional cardiovascular risks, including menopause, pregnancy related conditions and gynaecological conditions. However, these risks are still not well understood. Inclusion in trials where these risk factors are collected and analysed will improve our understanding.
What are the potential benefits for public health if more women are involved in trials?
We should ultimately be aiming for involvement in trials to better reflect our society. Women are still underrepresented - and women from ethnic minority and low socioeconomic backgrounds even more so - at all levels from funders to researchers, to clinicians to patients. Addressing this will improve the health of all.
More women participating in trials will improve understanding of cardiovascular risk, potentially helping to address the under-diagnosis of cardiovascular disease in women.
What’s been tried before to improve gender equity in clinical trials?
There has been increased focus on gender equity in the management of clinical trials in recent years. This has included increasing diversity among funder panels, trial committees and researchers, and so some progress has been made.
Publishers are also requesting information on equity, diversity and inclusion which has helped to highlight work that really needs to be done at the beginning of trials – not just the end.
Researchers involve patients, carers and the public in designing and running trials – known as patient and public involvement (PPI). As this improves in terms of gender and ethnic diversity, so do the numbers of women speaking about their experiences which gives us valuable insights we can learn from. We have successfully embedded PPI in our trial management groups and trial steering committees, and have developed a Research Advisory Group which is a diverse PPI group that advised on our cardiovascular trials.
What have you done to address the issue so far?
Within the Clinical Trials Unit at LSHTM, we have focused on making trials more accessible to all in recent years, collaborating with PPI groups to improve the patient experience.
We have improved communication with patients and diversified methods of communication; improved patient information, providing it in diverse ways such as videos and animations. In addition, we have simplified patient visits and follow-up.
We are also members of the Women in Cardiovascular Trials (WiCVT) group, which has been set up to investigate the barriers and facilitators to women’s participation in trials. The group is an international collaboration of patients, researchers, nurses and clinicians dedicated to improving representation of women in cardiovascular research.
What are you going to be investigating in your upcoming new trials?
Our next generation of trials due to start this summer are designed to be accessible to all. We have planned them with PPI input from the beginning to minimise barriers to women, people from ethnic minorities and low socioeconomic areas.
We are launching new trials that deal with issues more commonly seen in women, including female patients admitted with heart attacks and angina to investigate the best way to treat women in an acute setting. We will also be looking at other forms of heart disease that are more common in women than in men – heart failure with preserved ejection fraction (HFpEF) and Spontaneous coronary artery dissection (SCAD).
What actions are you taking to increase participation among women?
Women with lived experience have been involved with research design to ensure that the needs and priorities of women have been considered at every stage.
We plan to create patient information that is tailored specifically to women and properly takes into account the way in which women process the risks and potential benefits of taking part in the trial. We will discuss these with PPI groups before the trial starts.
We will use audio, video and written formats and allow participants sufficient time to make a fully informed decision. We will also provide information tailored to a patient’s partner and family, as in many cultures this forms a large part of the decision making process.
Practical steps include reimbursing for travel where needed and covering the cost of childcare or caring commitments for trial-specific visits, as this prevents many women from enrolling in trials. We will ensure that communication is flexible and there are different options for collecting information from patients that can fit better around their lives.
How will you measure the effects of new strategies and trial design?
We plan to start monitoring representativeness in real time. We are already doing that to some extent but we will now monitor sex, ethnicity and socioeconomic status in regular reports so that we can take action quickly and test the effectiveness of new approaches.
PPI members will sit on the Trial Management Groups, advising in real time on any issues affecting recruitment and help provide solutions to any new barriers we identify.
We plan to enhance monitoring of the enrolment process at participating hospitals, recording reasons for non-enrolment, along with sex (and potentially also ethnicity). We will also monitor usage of online resources to see which ones reach the most patients.
We will include small studies (SWATs) within the trial to evaluate how well some of the proposed solutions work at improving enrolment, presenting and publishing the results. That way we hope to contribute to improving recruitment of women across trials in future.
If successful, what kind of impact could this have in the future and what needs to happen next?
Funders need to accept that underrepresentation of women is unacceptable and consider it as a call to action. We need funding in order to test these strategies in a clinical trial setting.
Ultimately our job is to impact positively on public health. We do this in the CTU by answering clinical questions through trials. However, there are unanswered questions on women’s cardiovascular health that aren’t being considered as subjects for clinical trials because of a lack of confidence that these trials can be delivered.
If we can prove that recruiting women into clinical trials is feasible and achievable, it will provide a case for many more of these issues to be investigated in future.
The overall aim is to find new strategies and treatments that improve health and quality of life for all women.
Publication: Julie Sanders, Tim Clayton, Stacey Matthews, Sarah Murray, Lynn Laidlaw, Richard Evans & Rochelle Wynne, Strategies for the delivery of sex-based equity in cardiovascular clinical trials. Nature Reviews Cardiology. https://www.nature.com/articles/s41569-024-01025-x
If you enjoyed this article and would like to build a career in global health, we offer a range of MSc programmes covering health and data, infectious and tropical diseases, population health, and public health and policy.
Available on campus or online, including flexible study that works around your work and home life, be part of a global community at the UK's no.1 public health university.