Expert comment - University of Oxford vaccine announcement
23 November 2020 London School of Hygiene & Tropical Medicine London School of Hygiene & Tropical Medicine https://lshtm.ac.uk/themes/custom/lshtm/images/lshtm-logo-black.png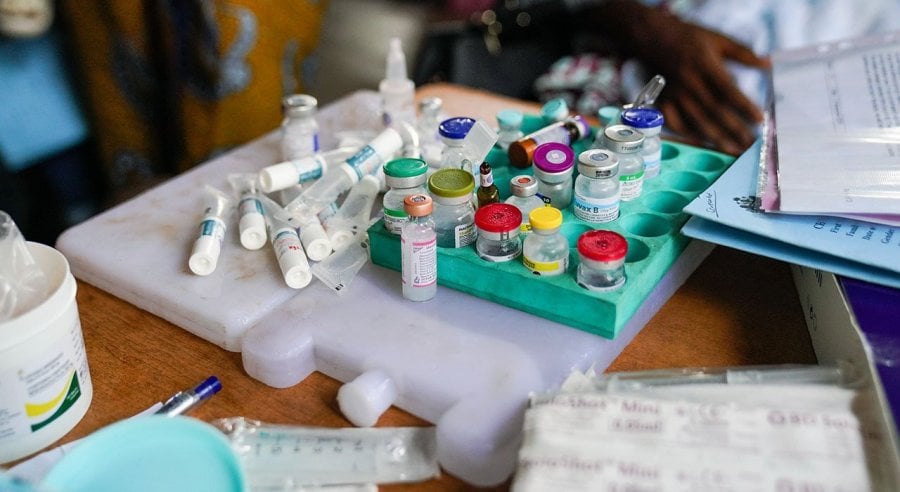
Vaccination vials sit on top of two freezer packs in an attempt to keep them cool in the humid climate of the outdoor ward in Basse Hospital, The Gambia. Credit: Louis Leeson/LSHTM
November 2020 has seen Pfizer and BioNTech announce that their ongoing Phase 3 trial of an mRNA vaccine shows 95% effectiveness against COVID-19, and Moderna report that its COVID-19 mRNA vaccine is 94.5% effective against the virus.
Now, the University of Oxford and AstraZeneca have announced that Phase 3 interim analysis indicates their vaccine provides between 62% to 90% protection from the virus.
Professor Peter Piot, Director of LSHTM, said: “2020 will be remembered for the many lives lost from COVID-19, lockdowns and the US election. Science should now be added to this list.
“Breakthroughs in science are nothing new, but the importance of the three announcements this month from Pfizer/Biontech, Moderna and today from University of Oxford-AstraZeneca cannot be overestimated.
“The pandemic has taken lives and livelihoods all around the world, turning our way of life upside down in ways we never thought would happen and crippling economies in the process.
“The only way to stop COVID-19 in its tracks is having multiple effective and safe vaccines that can be deployed all around the world and in vast quantities.
“I am very pleased to see 62% to 90% efficacy, depending on the doses used, for the Oxford-AstraZeneca COVID-19 vaccine. The encouraging news is that greater protection at lower dose may mean that more people can be vaccinated with the same amount of vaccines, and that this vaccine can be stored easily at regular fridge temperature. This will also help with equitable access all over the world. I eagerly await to see the data and their implications for deployment of vaccines against COVID-19.
"However, there are still many hurdles to cross before this pandemic is under control and we can return to normal life. Most importantly, we must all continue to follow the rules for safe behaviour to suppress the spread of SARS-CoV-2 and save as many lives as possible until we see the full and widespread impact of vaccination. At the same time, urgently addressing vaccine hesitancy is vital.
“However, November 2020 looks set to be the month that humanity developed the tools to turn the tide against this devastating virus.”
Stephen Evans, Professor of Pharmacoepidemiology at LSHTM, said: “This is good news, even if not as exciting as the news from the mRNA vaccines.
“It is likely that on one dosing regime (from my calculation based on information in the press release rather than what AZ said directly) is that with the smaller group (half/full) there were 3 cases of confirmed COVID-19 in the 2741 giving the vaccine and 29 cases in about the same number given the control (a Meningitis vaccine or placebo). This gives an efficacy close to 90% as reported. In the two full dose regime there were probably 27 cases of Covid in the vaccine group out of 8895 given it, while there were probably 71 cases in the control group. This gives an efficacy close to 62% as reported and combining the data across both groups gives an average of 70.2%. There are statistical uncertainties in each of these numbers and the overall results has a lower bound of over 50% which was a criterion mentioned as being of importance to regulators. There is little doubt that these data meet the criteria, based on numbers alone, for regulatory approval. Regulators will scrutinise all the detailed data before giving approval.
“The really good news is two-fold. Firstly, the vaccine can be stored in ordinary refrigerators, which helps in high-income countries but is of enormous importance for low-income countries. Secondly, the manufacturers and Oxford are committed to making it as easy as possible, through the WHO COVAX agreements, for the vaccine to reach low- and middle income-countries with their pledge to provide the vaccine at cost to such countries not just for the duration of the pandemic but indefinitely.”
If you enjoyed this article and would like to build a career in global health, we offer a range of MSc programmes covering health and data, infectious and tropical diseases, population health, and public health and policy.
Available on campus or online, including flexible study that works around your work and home life, be part of a global community at the UK's no.1 public health university.