Malaria Vaccine: A hope for Africa?
13 July 2023 London School of Hygiene & Tropical Medicine London School of Hygiene & Tropical Medicine https://lshtm.ac.uk/themes/custom/lshtm/images/lshtm-logo-black.png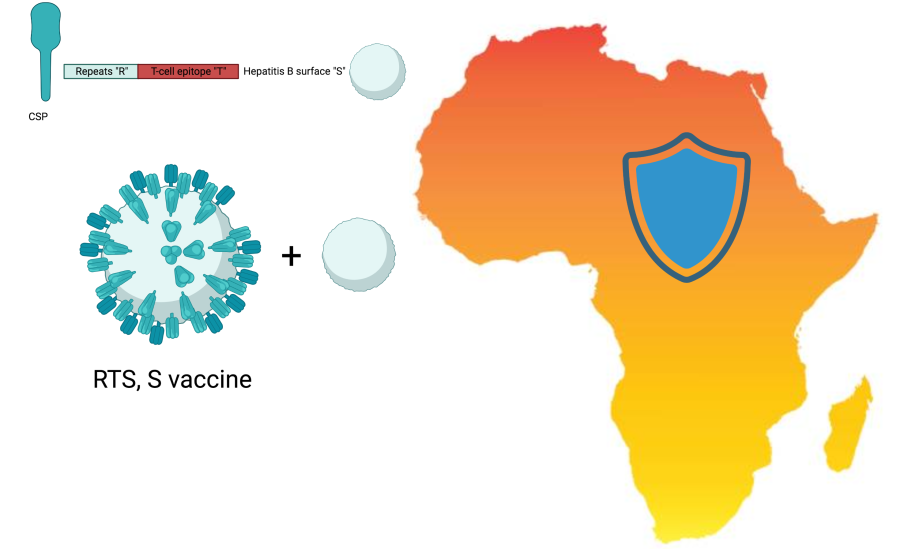
Twelve countries across Africa are set to receive 18 million doses of the first-ever malaria vaccine over the next two years. The vaccine roll-out marks a step forward in the fight against a disease that caused 435,00 deaths in 2017. A step culminating from more than half a century of research to find a vaccine against a cunning parasite. The global community is eagerly anticipating with high hopes a bright future of disease control in Africa.
The quest for a malaria vaccine began in the 1960s, inspired by the remarkable success of vaccines against polio, measles, diphtheria, and tetanus. However, researchers soon found themselves facing a challenging disease compounded by the complex biology of a parasite. Advancements in genomics, immunology and genetic engineering expanded our understanding of the life cycle of the parasite and its genetic diversity. These breakthroughs helped to unveil the Achilles heel of the parasite the circumsporozite protein (CSP), where the first malaria vaccine, RTS,S, will target.
CSP is a major surface protein on the malaria sporozite, cells that infect our liver, and its repeat regions constitute the “R” in the RTS,S vaccine name, while the “T” stands for the T-cell epitope of the CSP antigen (a target that our immune cells recognise). The CSP fragment is genetically fused to a surface antigen from Hepatitis B virus “S”. A second “S” stands for unfused Hepatitis-B surface antigen that spontaneously fuses to the RTS component. Therefore, the final structure of the vaccine resembles a virus. Developed over three decades by GlaxoSmithKline (GSK) in partnership with PATH and a network of African research centres, the vaccine is given in three doses, followed by a booster dose after 20 months. Phase III clinical trials enrolled 15,459 children at 11 locations in seven African countries showed that the vaccine is 56% effective over one year and 36% effective over four years.
Excitingly, ground-breaking research led by Sir Brian Greenwood at LSHTM, showed that the administration of RTS,S vaccine was on par to chemoprevention (antimicrobials) in preventing uncomplicated malaria. The study suggests that seasonal vaccination with RTS,S vaccine could be used as an alternative when resistance to chemoprevention drugs emerges.
Antimicrobial resistance in malaria is a major health concern. At LSHTM, research groups work tirelessly exploring mechanisms of malaria resistance to antimicrobials, understanding how malaria parasites escape rapid diagnostic tests, investigating the infection biology of a zoonotic malaria parasites, and developing of molecular tools to track malaria parasites.
Our latest Q&A with Dr Khalid Beshir, a genomic epidemiologist and winner of the AMR Centre Publication Prize 2023, sheds light on how malaria parasites escape rapid detection tests, and answers many more exciting questions about the world of a clever yet deadly parasite. You can watch his AMR seminar, Antimalarial resistance in Africa, here.
If you enjoyed this article and would like to build a career in global health, we offer a range of MSc programmes covering health and data, infectious and tropical diseases, population health, and public health and policy.
Available on campus or online, including flexible study that works around your work and home life, be part of a global community at the UK's no.1 public health university.