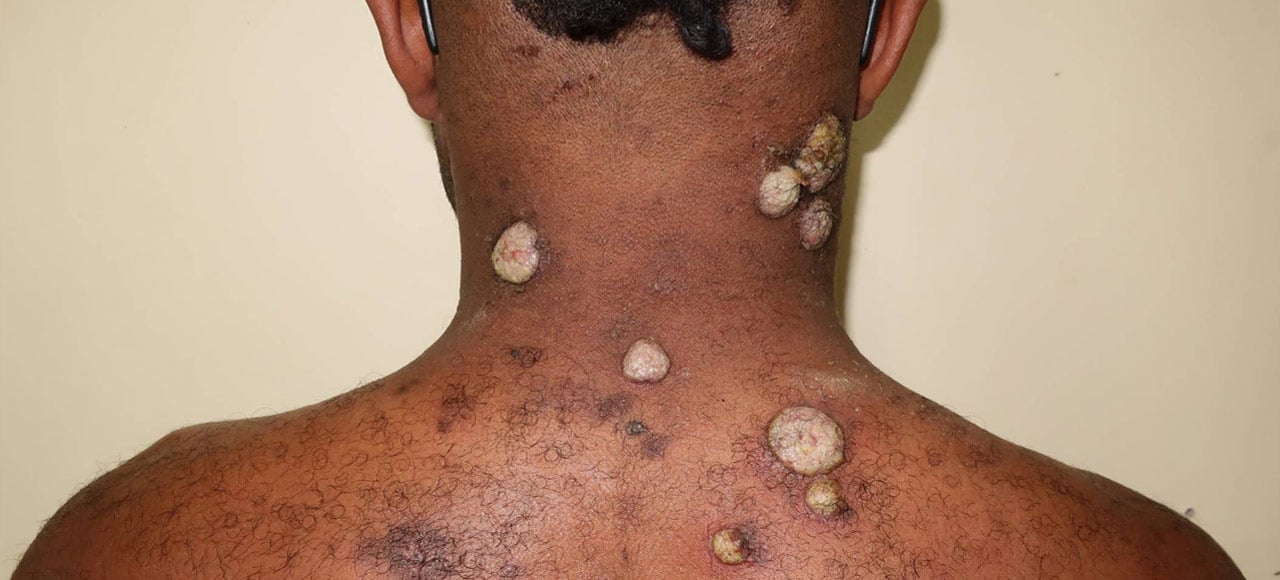
This project is part of the EDCTP2 programme supported by the European Union. The LAMP4Yaws study’s primary aim is to perform a diagnostic evaluation of the Treponema pallidum, Haemophilus ducreyi loop mediated isothermal test (TPHD-LAMP) for yaws. Additional multidisciplinary sub-studies will aid our understanding of yaws epidemiology and transmission, and assess the feasibility and cost-effectiveness of the TPHD-LAMP assay for use in yaws eradication campaigns and national surveillance programmes.
The LAMP4Yaws consortium consists of LSHTM (coordination centre); institutes in Europe (designing and refining the TPHD-LAMP assay and leading the development of an external quality assurance scheme); and in West and Central Africa (Sample collection and diagnostic evaluation).
The main objectives of the LAMP4yaws project are:
- To conduct multi-country case searches and epidemiological analysis of risk factors for yaws.
- To complete an evaluation of the TPHD-LAMP assay compared to the gold standard nucleic acid amplification test for the diagnosis of yaws-like lesions
- To use molecular tools and next-generation sequencing to understand the aetiology of yaws-like lesions, and optimise sequencing tools for point of care use.
- Conduct a health economic and social science evaluation of healthcare worker and community knowledge-attitudes and practices in relation to yaws and related diseases, and their diagnosis.
- Capacity building- a major aspect of this project is to strengthen within country capacity for conducting high quality clinical research.
We hope that the LAMP4Yaws study will ultimately result in WHO’s implementation of the TPHD-LAMP test for yaws as one of the key diagnostic tests in yaws eradication and post-eradication surveillance.
LAMP4Yaws is funded by the European and Developing Countries Clinical Trials Partnership and is a collaboration between partners in Cote D’Ivoire, Cameroon, and Ghana, where the field activities will take place, and partners in Europe involved with development and refinement of the TPHD-LAMP assay.
Meet the team
- Dr Michael Marks – Chief Investigator, LSHTM
- Dr Emma Harding-Esch – Co-investigator, LSHTM
- Becca Handley – Study coordinator, LSHTM
- Dr Nadine Borst, Albert-Ludwigs-Universität Freiburg
- Lisa Becherer, Albert-Ludwigs-Universität Freiburg
- Dr Tania Crucitti, Centre Pasteur du Cameroun
- Dr Orial Mitjà, Fundación Privada de Lucha contra el sida
- Dr Camila González Beiras, Fundación Privada de Lucha contra el sida
- Dr Sascha Knauf, The Friedrich Loeffler Institute, Federal Research Institute for Animal Health (FLI)
- Professor Solange Ngazoa, Institut Pasteur de Cote D’Ivoire
- Dr Mohammed Bakheit, MAST diagnostics
- Professor Kennedy Kwasi Addo, University of Ghana, Noguchi Memorial Institute for Medical Research
- Dr Sara Eyangoh, Centre Pasteur du Cameroun
See where we work for more information about our collaborating partners.
In-country partners
Cameroon – Centre Pasteur de Cameroun
The Centre Pasteur du Cameroun (CPC) is a technical body of the Ministry of Public Health of Cameroon. This institute was created in 1959 in Yaoundé and includes an annex in Garoua that was created in 1985 and another one in Douala created in 2004. The CPC operates directly under the umbrella of the Ministry of Public Health and Finances. The CPC is a member of the Institute Pasteur International Network (IPIN) with whom it shares the main mission: the containment of infectious diseases in Cameroon and also in the sub regions. The team in Cameroon is lead by Dr Tania Crucitti and Dr Sara Eyangoh, with Dr Serges Tchatchouang coordinating the laboratory work and sample collection, and Dr Patrick Awonda leading the social science studies. Josiane Balla is the administrator for the project. CPC is also coordinating the laboratory capacity building package of the project across the three study countries.
Cote D’Ivoire - Institut Pasteur de Cote D’Ivoire
The Pasteur Institute of Côte d'Ivoire (commonly known as IPCI) is a public industrial and commercial establishment (EPIC) directly placed under the supervision of the Ministry of Higher Education and Scientific Research of Côte d'Ivoire. The IPCI was created in 1972. The institute is located on two sites: Cocody (inside the CHU de Cocody) and Adiopodoumé (route de Dabou), in Yopougon. The missions are research, training, diagnosis and epidemiological surveillance. Since its creation, the IPCI has put its expertise at the service of the Ivorian populations and those of West and Central Africa. Surveillance missions in the field through the creation of different National Reference Centers (NRC) in the country under the Ministry of Health. IPCI is the national reference laboratory for disease surveillance in Cote d’Ivoire. In Cote d’Ivoire Professor Solange Ngazoa will be leading the study, Dr Kouadio Aboh Hugues will be coordinating the field work, and Dr Kouamé Mireille Sina will be coordinating the laboratory work and Sylla Aboubacar will be running the diagnostic tests within the lab. Dr Adringha Tano leading the social science projects. Soro Aoussata is the administrator for the project.
Ghana – Noguchi Memorial Institute for Medical Research
The Noguchi Memorial Institute for Medical Research (NMIMR) is Ghana’s leading biomedical research institute and is based at the University of Ghana located in the Ghanaian capital, Accra. The Institute was established in 1979 through combined efforts by the former Dean of the University of Ghana school of medicine, Prof. E. O. Easmon and Prof. Kenji Honda of Fukushima school of medicine in Japan. The main mission of NMIMR is to improve the health and wellbeing of Ghanaians and mankind through focused and relevant quality biomedical research, human resource development and support of national public health activities. In Ghana the study PI is Professor Kennedy Kwasi Adoo, Head of the Department of Bacteriology, he will be supported by Dr. Laud Anthony Basing, postdoctoral fellow at the Department of Bacteriology, who will coordinate the study and Dr. Daniel Arhinful, Senior Research Fellow at the Department of Epidemiology, who will lead the social sciences work.
Other collaborators
Albert-Ludwigs-Universität Freiburg
1. Multi-country case searches and epidemiological analysis of risk factors for yaws:
- Whilst performing active case finding of participants with yaws for the diagnostic evaluation we will also collect information on household demographics and water, sanitation and hygiene (WASH) variables to see which factors are associated with yaws infection. This could be valuable when implementing future eradication campaigns, both in-country and worldwide.
2. Performance evaluation of the TPHD-LAMP assay compared to the gold standard nucleic acid amplification test for the diagnosis of yaws-like lesions:
- For the diagnostic evaluation we will collect lesion swabs samples from patients we think have yaws based on their clinical presentation and serological point of care test results. We will collect two swabs, one of which we will send to the local laboratory where specially trained laboratory technicians will use the new TPHD-LAMP test to looks for yaws-causing bacteria. The other swab will be sent to the national reference lab in each country where the gold standard qPCR will be used. The accuracy of the new TPHD-LAMP test will then be determined by comparing the results to the PCR test. We will also use the Treponema pallidum macrolide resistance (TPMR) LAMP to see if the test is able to detect azithromycin resistant Treponema pallidum.
3. Utilising molecular tools next-generation sequencing methods to understand the aetiology of yaws-like lesions:
- From a subset of participants, we will collect additional swabs in order to discover more about causes of yaws-like lesions. Previous studies have shown that T. pallidum and H. ducreyi are often not detected in lesions’ samples and the cause of the lesions often goes unknown. We will use specific PCRs and metagenomic sequencing to discover which other pathogens may be responsible.
- We will perform further investigations into the role of H .ducreyi as a cause of yaws-like lesions.
- We will use whole genome sequencing to learn more about the epidemiology of yaws and track transmission of any resistance strains found.
- We will aim to optimise the Oxford Nanopore Minion sequencing for detection of T.p.pertenue, H. ducreyi, and macrolide resistance strains of both pathogens, in the field.
4. Health economic and social science evaluation of healthcare worker and community knowledge-attitudes and practices in relation to yaws and related diseases, and their diagnosis:
- Our diagnostic evaluation will be important for demonstrating if the TPHD-LAMP test’s performance is good enough when deployed in yaws-endemic countries. However, it is very important that a test adopted for a disease is easy to use, cost-effective, and well perceived by patients, healthcare workers and laboratory staff. Therefore, we will conduct a range of social science and health economic studies to learn more about people’s existing knowledge of yaws, and to determine if the TPHD-LAMP test will be acceptable, feasible and cost-effective for use in yaws eradication campaigns and on-going surveillance.