
A farmer stands in an indoor chicken coop, surrounded by chickens and reaching into a wooden hatchery
Selecting Efficient Farm-level Antimicrobial Stewardship Interventions from a One Health perspective (SEFASI)
Using participatory stakeholder elicitation, health economic modelling, and multi-criterion decision analysis to rank antimicrobial stewardship interventions from a One Health perspective in England, Senegal and Denmark.
We are a consortium of JPIAMR funded researchers aiming to provide a ranking of farm-level interventions to control antibiotic resistance from a One Health perspective, taking into account human and animal health impacts and costs.
The SEFASI project aims to create a range of outputs which can be used by future researchers and policymakers in the interest of open-source science.
Recent updates
By Nichola Naylor
Events
Newsletter
Contact us
Selecting Efficient Farm-level Antimicrobial Stewardship Interventions from a one health perspective (SEFASI)
We are a consortium of JPIAMR funded researchers aiming to provide a ranking of farm-level interventions to control antibiotic resistance from a One Health perspective, taking into account human and animal health impacts and costs.
We aim to do this by applying and expanding our Agriculture-Human-Health-MicroEconomic (AHHME) model, with its epidemiology and economic modules to three key settings: England, Senegal and Denmark.
In January 2022, the “Selecting Efficient Farm-level Antimicrobial Stewardship Interventions from a One Health perspective” (SEFASI) project was started; with the London School of Hygiene (LSHTM), the International Livestock Research Institute (ILRI), the University of Copenhagen and the UK Health Security Agency (UKHSA) working together to achieve the overall aim of:
To provide a ranking of farm-level AMU interventions, across a One Health perspective, for different AMU and AMR scenarios, utilising evidence on the epidemiological and economic impacts of interventions at the farm, healthcare system and wider economic levels to evaluate the cost-effectiveness of interventions. This is within the context of Denmark, England and Senegal.
Our concept
We have developed an approach to analysing AMR interventions from a One Health perspective, which informs all SEFASI research.
This involves first considering the range of AMR-related interventions available, across human, animal and environmental health. The approach then considers which stakeholders are present across these sectors, as well as all of their objectives and interests. Then, we model the impact of each of these interventions on stakeholder objectives over time. Finally, these outcomes are weighted based on their importance to the various stakeholders, and the available interventions are ranked holistically using the weighted average of the outcomes.
View our infographic on economic evaluations of One-Health interventions.
Our work packages
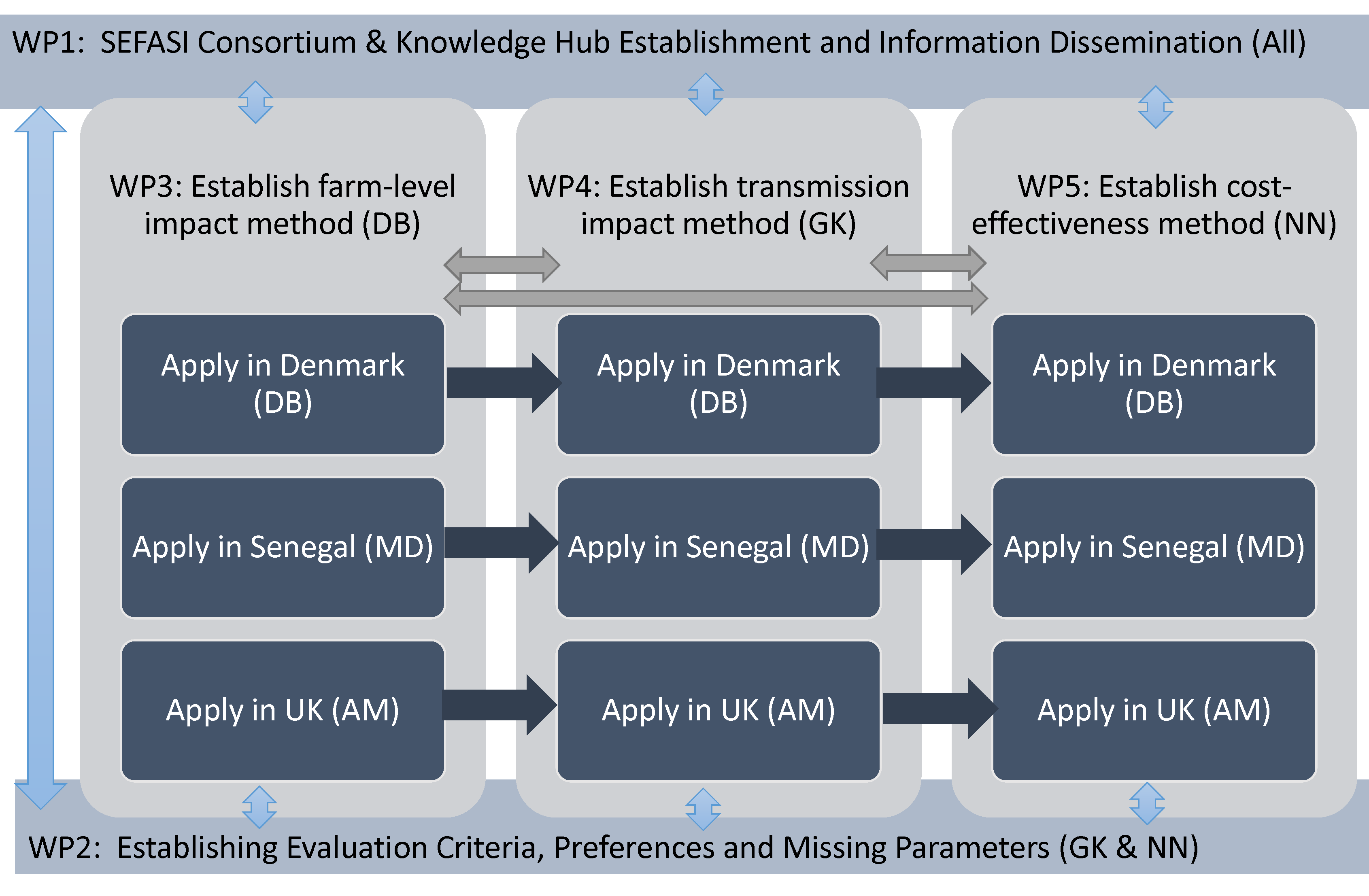
The SEFASI project will be carried out across five stages, or Work Packages (WP). In the first (WP1), we will bring together experts and stakeholders from our three country settings, as well as globally, to form the SEFASI Knowledge Hubs. WP2 involves organising workshops with these stakeholders, and eliciting their opinions to establish evaluation criteria and to find parameter values for future analysis. In work packages 3-5, we will model the impact of AMR interventions on farms, model the transmission of AMR and the impact of interventions on human health, and finally use these outputs to model the holistic cost-effectiveness of the interventions considered, across the our three country settings.
Our team
Our Consortium Co-Directors (Dr Gwen Knight (PI) & Dr Nichola Naylor (Deputy PI)), and Institutional Principal Investigators (Dr Dagim Belay, Dr Michel Dione & Dr Javier Guitian) are working with colleagues across institutions and regions to deliver the goals of SEFASI.
Also on the team are Eve Emes at the LSHTM and Ross Booton at Imperial College London. Joshua Aboah and Adiouma Faye at ILRI, and Yechale Endalew from University of Copenhagen, are also joining the SEFASI consortium to collaborate on the modelling efforts.
Recent updates
By Nichola Naylor
Events
Newsletter
Contact us
The SEFASI project aims to create a range of outputs which can be used by future researchers and policymakers in the interest of open-source science. Aside from the creation of the SEFASI Knowledge Hubs, we aim to form a library of up-to-date literature on agriculture and AMR, and to make available the data used in our research so that it can be repurposed for future research. Among our most important outputs are the reusable models that we create as part of the project, and the journal articles that will be published as part of our research.
Publications
How does antibiotic consumption in humans and animals link to AMR at the national level? And how best to estimate this relationship? - an opinion piece with recommendations for future research
This paper argues that we do not know enough about the relationship between antibiotic use and antibiotic resistance, at the population level, to inform health-economic decision making. We emphasise the need to estimate an actual number of resistant infections averted from a given change in national antibiotic use, and that this concern has been given insufficient attention in the literature. We highlight the potential of regression models using population-level data to bridge this gap, and outline a robust methodology for doing so in future research.
Country case study: how has Denmark’s Yellow Card Initiative affected antibiotic use in pig farms?
Denmark is considered a world leader in antimicrobial stewardship. Notably, the Yellow Card Initiative, introduced in 2010, places quantitative restrictions on antibiotic use in farms. While the Initiative has greatly reduced antibiotic use, it is uncertain what effect it has had on farms’ economic performance. Using panel m-order conditional efficiency analysis of Denmark’s pig farms, we find that the Initiative has been associated with a small reduction in revenue efficiency (0.15 percentage points) and a small reduction in cost efficiency (0.03 percentage points) on the average farm.
Using system dynamics modelling to map the causal networks of antibiotic resistance in Senegalese poultry production
Antibiotic use, the development of resistance, and the economics of livestock production form a complex causal network which we need to understand in order to design and implement AMR interventions in livestock farms. We held a workshop in Dakar involving stakeholders from public policy, academia, agriculture, hospitals, and the pharmaceutical industry. We used their inputs to create a causal loop diagram of the poultry production AMR system in Senegal. The model that we developed allows us to identify potential points of intervention. Once parameterised with real data, it can be used to quantify the estimated economic and epidemiological impact of prospective interventions at the farm level.
Country case study: what drives antibiotic use in poultry production? The case of Dakar and Thiès' peri-urban chicken farms
This paper is one of several investigations done using the AMUSE survey tool, which aims to collect information about knowledge, attitudes and practices relating to AMR in smallholder livestock farms. In order to manage or reduce antibiotic use in farms, we first must understand why farmers use antibiotics in the first place, and if there are any strategies which can reduce reliance on them. Better vaccination, better biosecurity, and better awareness of AMR issues have all been proposed as ways of reducing the need for antibiotics in farms. Using survey data from semi-intensive poultry farms in Dakar and Thiès, we investigate the extent to which these factors drive antibiotic use, and if they can help to mitigate any productivity loss from reducing antibiotic use. we found evidence that biosecurity improvements and AMR awareness-raising could encourage prudent use of antibiotics, and that biosecurity and vaccination could to some extent replace antibiotics as productivity-enhancing and disease management tools in broiler farms.
[preprint] Creating better framework for understanding how antimicrobial use affects society across all sectors
This paper (in preprint) creates a theoretical framework which can be used to estimate the health-economic effect of antimicrobial use on all sectors of society. This includes all of the effects considered in AHHME (with some tweaks and improvements), as well as the indirect effects on population health via food security, economic security, and constrained healthcare resources. This framework proposes a mixture of statistical and mathematical models with much lower data requirements than previous models. It can be applied to a range of AMR issues outside of agriculture, and we hope to work with collaborators in Zambia and Malawi in the coming months to estimate the national impact of AMR in these countries using this framework.
The agriculture human health microeconomic approach - a model for estimating the holistic cost-effectiveness of antimicrobial resistance interventions in livestock production
This paper introduces and explains our Agriculture Human Health Microeconomic (AHHME) model. AHHME uses mathematical modelling to estimate the societal effect of agricultural antibiotic use interventions. It considers the impact on: agricultural productivity, human labour productivity, life years lost to illness, and healthcare sector costs. By getting a more holistic estimate of societal impact, we hope to more accurately rank, design, and prioritise AMR interventions in agriculture. It is our goal for researchers and policymakers globally to use AHHME to estimate the impact of prospective interventions in their own contexts, and to improve and adapt the model code.
Country case study using the AMUSE survey: how do farm practices and antibiotic use habits affect disease incidence in Uganda’s smallholder livestock farms?
This paper is one of several investigations done using the AMUSE survey tool, which aims to collect information about knowledge, attitudes and practices relating to AMR in smallholder livestock farms. Prophylactic use of antibiotics in livestock is often seen as inappropriate and is targeted for reduction as part of antibiotic stewardship initiatives. However, these antibiotics may guard against animal disease and may be economically important for smallholder livestock farmers. Before reducing them, we need to determine the extent to which they guard against animal disease, and to identify other farm practices which can guard against disease and could therefore make such antibiotic use reductions safer. In smallholder livestock farms in Uganda, we found that prophylactic antibiotic use did guard against disease. While results varied by livestock species, we identified expanded access to animal health services and the use of prophylactic vaccination as ways of making prophylactic antibiotic use reduction safer.
Determinants of animal disease and nontherapeutic antibiotic use on smallholder livestock farms
This paper is one of several investigations done using the AMUSE survey tool, which aims to collect information about knowledge, attitudes and practices relating to AMR in smallholder livestock farms. A major goal of agricultural antimicrobial stewardship is to reduce antibiotic use (ABU) in livestock farms. However, farmers cannot simply be forced to reduce their ABU, given that doing so may worsen animal disease and given the availability of markets for illegal antibiotics. Instead, we must understand what factors can encourage farmers to reduce their antibiotic use on their own terms, and which factors can help to manage animal disease and therefore make antibiotic use reduction safer. In smallholder livestock farms in Burkina Faso, we found that going to a public veterinarian could encourage antimicrobial stewardship and reduce disease, relative to other animal health service providers. We found that formal education may also reduce animal disease.
[upcoming] Country case study: drug-resistant Campylobacter infections and antibiotic use in humans and poultry in the UK
In this paper, we use national-level data to investigate the ecological relationship between antibiotic use (in humans and poultry) and the rate of antibiotic resistance in Campylobacter in the UK. We find that antibiotic use does influence the rate of resistance in human Campylobacter infections, but is not the main determinant of it. For antibiotic use in human health, we find evidence that the relationship between use and resistance weakens over time as resistance builds up. We conclude that reductions in antibiotic use alone are unlikely to bring down the rate of AMR in Campylobacter to desirable levels, and that more attention should be paid to socioeconomic drivers of resistance, transmission factors, and alternative and complementary therapies such as vaccination and bacteriophages.
Country case study: The contribution of animal antibiotic use to antibiotic resistance in human infections: Panel evidence from Denmark
Taking advantage of extensive surveillance data available from DanMap, we are currently running panel regressions across a number of drug-pathogen combinations to investigate how the rate of antibiotic resistance in humans relates to the AMR prevalence and use of antibiotics in food animals in Denmark. This will give insight into the extent to which livestock AMU influences human AMR in Denmark, and can help to estimate the potential benefit of livestock antibiotic stewardship interventions in Denmark and elsewhere.
Conference and workshop outputs
SEFASI consortium outputs are regularly shared with researchers, policymakers and stakeholders, and engaging this participatory process is one of our main objectives. So far, this has meant that:
The framework presented in this preprint was presented and discussed at the JPIAMR New Perspectives on Bacterial Drug Resistance workshop on 9 June 2022
From 26-27 September 2022, the SEFASI consortium hosted a workshop in Dakar where we presented our ongoing work to a range of researchers, policymakers and stakeholders from across the One Health - AMR spectrum, starting a two-way conversation with stakeholders about priority-setting in AMR research and policy
We presented the AHHME and AHHME-B models at the Strengthening Implementation of National Action Plans through a One Health AMR Full Economic Evaluation (SNAP-ONE) workshop in Kariba from 3-5 October 2022. We showed how these models, and the framework presented in this preprint, can help to estimate the holistic effect of AMR and AMR policies with minimal data requirements
On 16 November 2022, we held a workshop to discuss how best to model the ecological relationship between antibiotic use (in humans and poultry) and the rate of AMR in UK Campylobacter infections. This workshop involved stakeholders from the British poultry industry, the British Poultry Council, the UK Health Security Agency, the UK Animal and Plant Health Agency, the UK Veterinary Medicines Directorate, the London School of Hygiene and Tropical Medicine, and the Royal Veterinary College
On 1 March 2023, we held our first SEFASI Knowledge Hub workshop, involving stakeholders across the One Health spectrum from England, Denmark and Senegal, as well as international stakeholders. We presented our research so far, and elicited stakeholder inputs on key areas for future research and intervention in One Health AMR
In April 2023, we presented the AHHME and AHHME-B models at the International Conference on One Health Antimicrobial Resistance (ICOHAR) in Copenhagen
In September 2023, we visited the National University of Singapore to present the AHHME and AHHME-B models as part of the One Health Antimicrobial Resistance Research Programme (OHARP) International Stakeholder Engagement Workshop on AMR Modelling
In January 2024, we had a SEFASI workshop in London attended by representatives from all 3 countries.
In September 2024, Eve Emes presented her work for SEFASI at the 8th World One Health Congress in Cape Town.
Models
The SEFASI GitHub Page
The SEFASI GitHub page is currently under construction, and when completed will contain all coding resources from the numerous projects which make up SEFASI.
The Agriculture Human Health MicroEconomic (AHHME) Model
The purpose of the model
Antimicrobial stewardship policies in agriculture affect every sector of society. Reducing antibiotic use in farms may reduce farm productivity and hurt farmers’ incomes, and this effect could be mitigated by improving farming practices in tandem. Implementing these interventions will incur a cost to the public sector. If reducing antibiotic use in agriculture reduces the number of people infected by drug-resistant bacteria, then this will prevent illness. Few illnesses also means fewer long-term sequelae, and fewer deaths. This, in turn, reduces the burden on the healthcare system, and means that fewer people are left unable to work (both in paid and unpaid work).
The Agriculture-Human-Health-Micro-Economic (AHHME) model takes all of these outcomes into account, and compares them in like terms. This gives us the cross-sectoral cost-effectiveness of hypothetical antimicrobial stewardship policies in agriculture. Depending on the information available, this can take the form of net monetary benefit of a proposed policy, or a threshold price (the maximum amount that we should be willing to pay to implement that policy).
Applications of the model
The AHHME model is flexible to a number of different methodological assumptions, types of intervention, and country characteristics. By customising these parameters, the model can be applied to any antimicrobial stewardship policy in any setting, and with various scopes of analysis.
In a published paper, we apply the model to a range of hypothetical antimicrobial use interventions in high-, middle- and low-income contexts (see ‘Publications’ for information on this and other SEFASI outputs).
On our public GitHub repository, we have included the code used to create and run the AHHME model in R. It also includes an example of how to adapt the model to any context and purpose. Our hope is that researchers and policymakers can use AHHME to model the cost-effectiveness of prospective agricultural antimicrobial stewardship policies in their own countries. Comparing outcomes in like terms across sectors will allow AMR policies to be compared to other, unrelated policy alternatives, allowing AMR to be correctly prioritised on the national policy agenda.
We have also created an interactive Shiny App which allows users to edit model inputs in a more straightforward way. This is intended to allow researchers to familiarise themselves with the model, and for exploratory analysis of potential interventions.
The AHHME-B Model
We have also developed the Agriculture Human Health MicroEconomic - Burden (AHHME-B) model, a compartmental mathematical model based on the AHHME model which estimates the holistic burden of antimicrobial resistance at the population level. It considers the same types of outcomes (agricultural productivity, labour productivity, healthcare costs, and life years lost to disease). However, instead of comparing an ‘intervention’ and ‘non-intervention’ scenario, it compares the existing situation to a series of counterfactuals in order to estimate both the associated and attributable burden of AMR at the national level. The scope can range from a single resistant pathogen strain to all resistant strains together. The model code can be found on GitHub and you can also access the interactive app. We are collaborating with researchers in the SNAP-ONE consortium to use this model to estimate the holistic burden of AMR in Zambia and Malawi, and are planning to estimate the burden of drug-resistant Campylobacter and E. coli in the UK as well.
Recent updates
By Nichola Naylor
Events
Newsletter
Contact us
Background and rationale

Stakeholder participation has been recommended in the evaluation of interventions tackling One Health issues (Naylor, 2020). This includes stakeholders from across the One Health and antimicrobial resistance (AMR) system; from farmers, clinicians, veterinarians, ministers of sectors and/or international organisations, the pharmaceutical industry and the general public.
The overarching goal of the SEFASI Knowledge Hub is therefore to bring together a group of experts and stakeholders from across the One Health - agriculture - AMR spectrum, and to elicit their expertise with the aim of informing multi-criterion decision analysis which will allow us to rank and prioritise AMR policies.
Knowledge Hub aims and research questions
The aims of the Knowledge Hub are:
To utilise knowledge hubs for the expert elicitation of ranking of the importance of:
- the interventions, AMR prevalence and agricultural system scenarios of greatest interest, in relation to farm-level AMU,
- the impact outcome measures of most importance,
- stakeholder preferences in terms of the ranking of such outcome measures and
- missing input parameters critical for epidemiological and economic modelling of relevant interventions across One Health systems, within the three country case studies.
Knowledge hub research questions are:
What do stakeholders believe are:
- the interventions and AMR and One Health scenarios of greatest interest?
- the evaluation criteria of most importance?
- their preferences in terms of the weighting of such outcome measures? and
- missing input parameters critical for epidemiological and economic modelling of relevant interventions across One Health systems?
Our methods
Multi-criteria decision analysis (MCDA) is a formal process in which stakeholder preferences for the importance of different measures of intervention impact are used to weight impact estimates (e.g. cost-effectiveness of one intervention compared to others against equity impacts of intervention implementation) to allow for a final ranking of interventions. MCDA approaches have been used in zoonotic disease fields and AMR separately. These processes can (i) improve end-user use of research outputs and (ii) utilise the knowledge resource of stakeholders in understanding the importance of evaluation measures and unknown parameters in a formal manner.
Additionally, expert elicitation for the parameterisation of models where data are missing and/or need more context (for example for microbes where surveillance data are missing or for predicting pandemic-AMR scenarios) can be used in the parameterisation of infectious disease and economic evaluation models. Previously experts have been utilised to understand AMR and its burden, focusing more on AMR-burden data needs and quality.
In SEFASI, we are proposing to bring together groups of experts and stakeholders, across the One Health arena, in the form of “Knowledge Hubs”. We will develop membership of these Hubs through (i) local networks within the three participating countries, (ii) mapping of One Health areas and active determination of experts in each needed arena in each geographical area and (iii) approaching international organisations.
Initiation of Knowledge Hub
- Round 1: Intervention, scenario and outcome prioritisation
- Round 2: Missing parameter elicitation
- Round 3: Multi-criteria decision analysis
Knowledge Hub members
- Dr Jonathan Bastard (University of Colombia, USA)
- Dr Fiammetta Bozzani (LSHTM, London, UK)
- Prof. Cheikh Saad Bouh Boye (Honorary Dean FMPO UCAD, Honorary Rector UIDT THIES, President of Senegalese Society for Microbiology, Co-founder of African Vaccines Manufacturing Initiative (AVMI), International Coordinator of Vaccinology Training Degree, CEO AFRICASEPTIC SARL, Dakar, Senegal)
- Prof. Makhtar Camara (Titulaire des Universités, Laboratoire de Bactériologie-Virologie, CHNU A. Le Dantec, Dakar, Senegal)
- Dr Sara Danièle Dieng (Ecole Inter-Etats des Sciences et Médecine Vétérinaires (EISMV) de Dakar, Dakar, Senegal)
- Dr Yakhya Dieye (Institut Pasteur, Dakar, Senegal)
- Prof. Becaye Fall (Chef de Laboratoire, Hopital Principal, Dakar, Senegal)
- Dr Ousmane Faye (Country Project Director – Senegal, USAID Medecines, Technologies and Pharmaceutical Services (MTaPS) Program)
- Dr Amadou Gueye (Initiative Prospective Agricole et Rurale (IPAR) Dakar, Senegal)
- Dr Daouda Gueye (CEO, Société Sénégalaise pour le Développement de l'Elevage (SOSEDEL), Dakar, Senegal)
- Prof. Anette Loeffler (RVC London, UK)
- Dr Nubwa Medugu (Nile University of Nigeria)
- Prof. Dominic Moran (University of Edinburgh, UK)
- Dr Papa Abdoulaye Seck (Ministère de la Santé et de l'Action sociale, Dakar, Senegal)
- Dr Malik Orou Seko (Ecole Inter-Etats des Sciences et Médecine Vétérinaires (EISMV) de Dakar, Senegal)
- Dr Walter Ossebi (Ecole Inter-Etats des Sciences et Médecine Vétérinaires (EISMV) de Dakar, Senegal)
- Prof. Kristen Reyher (University of Bristol, UK)
- Dr Adam Roberts (University of Liverpool, UK)
- Dr Mouhamed Bachir Wilane (Haut Conseil National de sécurité Sanitaire Mondiale One Health, Dakar, Senegal)
Recent updates
By Nichola Naylor
Events
Newsletter
Contact us
The SEFEASI Consortium are conducting our research in three countries: England, Senegal, and Denmark.
- Denmark
-
Area lead
Dagim Belay
One Health AMR situation
Denmark has been monitoring both AMU and AMR in animals, food and humans since 1995 (Hammerum, 2007) and has been a pioneer in developing, implementing and assessing the impact of interventions to curb AMU with an early focus on animal populations. Interventions to reduce fluoroquinolone (2002, 2003) and cephalosporin (2010) use in food-producing animals, alongside systems to monitor antibiotic use in pig-production (Yellow Card in 2010), resulted in a marked reduction in the use of antibiotics across pig farms between 2010-2017 (Antunes, 2020). Denmark has combined this low use with being one of the largest pig producers in the world with a production of up to 32 million pigs a year, which is highly intensified and highly productive (DAFC, 2021). We expect that this country will have most agricultural sector evidence available for the development of our models: decades of reliable surveillance data, including antibiotic use at animal level (i.e., DANMAP reports), several research study publications and at least one case study conducted by FAO to explore the effectiveness of different interventions to curb AMU in Danish pork production (FAO, 2019). The focus on “food, food animals and humans” of the Danish action plan on AMR suggests that the environmental aspect may be less well analysed, but the Danish championing of a One Health approach gives confidence that data will be available going forward.
Upcoming and ongoing work
As part of the SEFASI project, we have published a paper using efficiency analysis to estimate the effect of Denmark’s ‘Yellow Card’ antibiotic stewardship initiative on the economic performance of Danish pig farms. We also aim to publish a regression analysis which uses Danish surveillance data to characterise the relationship between antibiotic use in different species and antibiotic resistance in humans at the population level. See our Publications section on the Outputs page to find out more.
- England
-
Area lead
Javier Guitian, Royal Veterinary College
One Health AMR situation
England has been a pioneer in the use and open-sharing of action plans and data on AMR prevalence within human infections (HMG, 2019), with National Action Plans since 2013. This impetus was reflected in the UK Prime Minister commissioning a review in 2014 of the global state of AMR (O’Neill, 2016). The UK has a well developed dairy and beef cattle sector. It is self-sufficient for beef production with approximately 9.36 million cattle contributing approximately £2.8 billion to the UK economy (NBA, 2021). Following the publication of the O’Neill report on AMR in 2016, a Task Force was created by the Responsible Use of Medicines in Agriculture Alliance (RUMA) to set up targets for reduction of overall and highest-priority Critically Important Antibiotics (HPCIA) use across different livestock sectors (RUMA, 2020). Led by the livestock industry through education and behaviour change interventions, a series of indicators are in place to monitor efficacy of these interventions on levels of AMU, adoption of best practices and impact on animal welfare.
The use of antibiotics at animal and farm levels are still unavailable for the whole of the national herd but data are available for antibiotic sales. These show that dramatic reductions (>40%) have been observed in the livestock sector between 2015 and 2019 (UK-VARSS, 2020), firstly in poultry, then pig and more recently in cattle. Sales of injectable and topical intramammary HPCIAs antibiotics for use in cattle in the UK has decreased considerably in recent years from 1.1 mg/kg (2015) to 0.26 mg/kg (2019) and from 0.33 mg/kg (2015) to 0.03 mg/kg (2019) representing a reduction of 77% and 91% of sales of these types of formulations, respectively. Further reduction targets have been established for the period of 2021-2024 (UK-VARSS, 2020). On the human side, data on antimicrobial consumption is available at the primary case and secondary care level from 2016/7 onwards (PHE, 2021), with national level data available from 2010 through the English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) reports. As antibiotics are not used on crops, there is little selective pressure in crop agriculture (Haynes, 2020). England now has a framework for understanding environmental antimicrobial resistance led by the Environment Agency (Singer, 2020) which has collated all datasets that could provide an environmental AMR risk assessment for consultation.
Upcoming and ongoing work
As part of the SEFASI project, we have done rounds of stakeholder elicitation with members of academia, public health policy and the poultry industry. We are using these inputs to publish a regression analysis which investigates the link between antibiotic use (in human health and poultry production) and the rate of antibiotic resistance in Campylobacter infections in the UK at the population level. See our Publications section on the Outputs page to find out more.
- Senegal
-
Area lead
Michel Dione
One Health AMR situation
Senegal is a leader in the African continent in terms of its approach to AMR control. It has had a National Program for the Control of Nosocomial Infections (PRONALIN) in place since 2004 (Ndoye, 2009) which has been effective in reducing infections and the transmission of multidrug-resistant bacteria associated with hospital care and a Directorate of Laboratories which set up in 2012 a national system for monitoring antibiotic resistance through laboratories (RNL/SN, 2013). Building on these, Senegal has adopted a wider "One Health" strategy, in support of the global health security agenda, to strengthen integration and intersectoral cooperation in the prevention, detection and response to threats of emerging infectious diseases, including zoonoses and AMR (Senegal Government, 2016). Specifically, a National Multisectoral Antimicrobial Resistance Surveillance and Control Action Plan (2018-2022) was prepared by the Ministry of Health and Social Action (Laboratories Directorate) in 2017 (Coll-Sec, 2020), with technical and financial support from WHO and FAO. Moreover, Senegal has the One Health National Platform composed of all actors involved in the control of zoonotic diseases and AMR including the 3 ministerial pillars of One Health, the private sector, the national and regional research institutions and development organisations. This platform is already supporting an ongoing initiative using a One Health approach to tackle AMR with FAO (FAO, 2020) and the Fleming Fund (2021). However much of the data needs to be aggregated and analysed.
The poultry population is estimated at 64,541,000 heads in 2016, an increase of 7.7% compared to 2015. This increase is attributable to the 11.0% increase in industrial poultry. In 2019, the turnover of the poultry sector is estimated at at least USD 0.4 billion. The share of industrial poultry has increased considerably to supplant beef in 2019: taking into account family poultry, which is estimated at about 13% of per capita consumption, makes poultry meat the first meat consumed per capita in Senegal, or 44% of the total (Ly, 2020). Most intensive poultry farming happens in peri-urban areas where animals receive a large amount of drugs such as antibiotics to fight diseases and boost growth, and compliance with the biosecurity norms of poultry production remains a challenge (Biagui, 2002). In addition, studies conducted on cattle, sheep and/or poultry meat have revealed the presence of antibiotic residues in their products; but the risk to humans has never been evaluated in such settings (Aambedji, 2004).
Upcoming and ongoing work
As part of the SEFASI project, we have published a regression analysis which investigates the determinants of antibiotic usage in peri-urban semi-intensive poultry farms in Dakar and Thiès.
Consortium member Joshua Aboah has also used stakeholder inputs to create a system dynamic model of AMR and poultry production in Senegal, similar to previous work based in Ghana, and to publish a paper expounding the model. This modelling project allows us to understand and model how farmers' behaviour interacts with micro- and macro-level variables, both in terms of production decisions and antimicrobial stewardship.
See our Publications section on the Outputs page to find out more.
Recent updates
By Nichola Naylor
Events
Newsletter
Contact us
- December 2024: Official end of UK funding for SEFASI.
- October 2024: Successful SEFASI consortium meet in Dakar to summarise outputs, discuss policy brief, human health data and next steps.
- September 2024: Presentation of SEFASI work at the 8th World One Health Congress in Cape Town.
- January 2024: Successful SEFASI consortium meet in London to discuss transmission modelling and AHHME.September 2023: AHHME and AMMME-B presented at the National University of Singapore
- January 2024: SEFASI will meet in person in London to hold an update meeting and to hold our second Knowledge Hub meeting (morning of 11 January).
- August 2023: AHHME paper AHHME: A model for estimating the holistic cost-effectiveness of antimicrobial resistance interventions in food animal production published in One Health
- August 2023: How farm practices and antibiotic use drive disease incidence in smallholder livestock farms: Evidence from a survey in Uganda published in One Health.
- 13 July 2023: paper Mapping the effect of antimicrobial resistance in poultry production in Senegal: an integrated system dynamics and network analysis approach published in Frontiers in Veterinary Science
- 18-20 April 2023: AHHME and AHHME-B models presented at the International Conference on One Health Antimicrobial Resistance (ICOHAR 2023) in Copenhagen
- 1 March 2023: first SEFASI Knowledge Hub workshop held
- 1 February 2023: paper Drivers of Antibiotic Use in Semi-Intensive Poultry Farms: Evidence from a Survey in Senegal published in Antibiotics
- 16 November 2022: stakeholder meeting held to discuss how best to model the ecological relationship between antibiotic use (in humans and poultry) and the rate of AMR in human Campylobacter infections in the UK
- 3-5 October 2022: AHHME and AHHME-B models presented at the Strengthening Implementation of National Action Plans through a One Health AMR Full Economic Evaluation (SNAP-ONE) workshop in Kariba
- 26-27 September 2022: SEFASI technical inception workshop for Senegal
- 25 August 2022: SEFASI update meeting - discussion of data sharing and current status of knowledge hub protocols and website
- 28 July 2022: SEFASI update meeting - discussion of how to integrate Josh's system dynamics modelling into the AHHME framework.
- 22 June 2022: SEFASI update meeting - lots of discussion after a presentation from Ross Booton of his mathematical modelling across One Health environments
- May 2022: First SEFASI affiliated publication from Dagim on "Does restricting therapeutic antibiotics use influence efficiency of pig farms? Evidence from Denmark’s Yellow Card Initiative". Congratulations Dagim!
- 5 May 2022: SEFASI update meeting - Michel presented data and analysis of the current situation in Senegal.
- 21 April 2022: SEFASI update meeting - Dagim presented his analysis and data of work in Denmark.
- 10 February 2022: SEFASI update meeting - lots of interesting discussion as to current status and ideas for discussion piece and next meeting
- 17 January 2022: SEFASI kick-off meeting held virtually: AHHME model presentation and discussion
- 1 January 2022: SEFASI officially starts
Recent updates
By Nichola Naylor
Events
Newsletter
Contact us
By Nichola Naylor
National Action Plans (NAPs) for Antimicrobial Resistance (AMR) highlight the intended objectives a country has in relation to tackling AMR, and gives an overview of how these objectives are intended to be met over a given time period. The World Health Organisation houses a library of NAPs, available here: Library of national action plans (who.int).
There are often calls for more epidemiological and economic evaluations of interventions aimed at tackling AMR, to subsequently lead evidence-based policy making in relation to future NAPs. In order to fill this gap in relation to farm-level interventions and their links with antimicrobial usage (AMU) and the subsequent impacts on AMR-associated costs, we first must understand what are the key interventions/categories of interventions we should be evaluating. To have a more detailed understanding of the landscape of priority farm-level interventions that could relate to AMU, from the perspective of policy makers, such NAPs were reviewed for the country case studies of SEFASI. Prior to the first SEFASI Knowledge Hub meeting (see blog here), we wanted to understand what potential interventions related to farm-level antimicrobial usage policies could be found in SEFASI case-study countries. The following documents were included for review:
- For Denmark: The Danish Veterinary and Food Administration's national action plan for antibiotic resistance in production animals and food (pdf)
- For England: Tackling antimicrobial resistance 2019–2024 - The UK’s five-year national action plan (pdf)
- For Senegal: Plan d'action national multisectoriel de surveillance et de lutte contre les résistances aux antimicrobiens (pdf)
They covered overlapping but slightly different timelines, with Senegal’s previous NAP used (covering 2018-2022), the UK’s NAP which is due for an update in 2024(covering 2019 - 2024) and Denmark’s finishing in 2023 (2021 - 2023). The specific NAP objectives that the interventions fell under, their direct description within the NAPs, the intended population of interest and mentioned measurements of impact were extracted. Whether these are related to more generic categorisations of farm-level interventions (e.g. biosecurity, vaccine, hygiene, stewardship), and whether these are considered AMU-sensitive or AMU-specific (reduced AMU indirectly or directly respectively), were then noted.
The related objectives pulled from these NAPs are available in Table 1, at the end of this blog. Note that additional information presented (such as intervention and population descriptions) might be subjective interpretation, or an umbrella-term/summary of the information provided within the NAPs - please refer to the NAP documents for specific details regarding each if in doubt.
Regarding target type, all countries had a mix of both types (Figure 1). Senegal and Denmark focus more on specific-AMU interventions predominantly, whereas the UK focuses more on AMU-sensitive interventions, in relation to AMU that's associated with farms. Box 1 presents an overview of specific interventions found across the NAPs. Examples of where an intervention would be classed as both would be if it mentions the promotion of infection, prevention and control measures and stewardship, within the same aim.
Figure 1: NAP Objectives: Antimicrobial Sensitive or Specific?
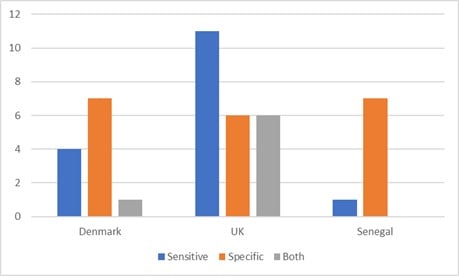
The intended impact described in each NAP ranged from very specific and measurable, to broader AMR goals. In terms of measurements of impact, antimicrobial use was the most common measure mentioned throughout the NAPs, though the Denmark NAP did also include monitoring of knowledge and awareness, hygiene and epidemiology of AMR. The UK NAP also mentioned that understanding cost-effectiveness at the farm-level will be key. For example, Denmark goals include to “achieve a reduction of 2 per cent per year (2019–2022) in the use of antibiotics for pigs, and maintain or reduce the use of antibiotics for other livestock species. Additionally, to maintain low use for production animals of those antibiotics that are critically important for treating humans (2019 level)”, which are specific and give baseline indicators and timelines.
Whilst Senegal had more general aims, with less stated impact measures, these aims and objectives were linked to exact, defined, activities linked to each objective. These also had a clear timeline of when these were planning to be executed over the period, with set budgets for each activity. For example, for “3.3. Strengthen the institutional and Regulatory process” in relation to the sale/provision of antimicrobials, an action of organizing 20 control missions including 10 in human health and 10 in animal health per inspection of antibiotic use (breeding and fishing) was listed.
Interventions found within the NAPs
- animal housing design and build
- group management
- minimum movement and mixing
- biosecurity and hygiene
- freezing quality colostrum
- guidance
- communication
- establishment and promotion of best practice
- animal health planning interventions
- promote vaccine use
- gene therapies
- nutrition
- targeted interventions based on animal disease data
- training; teaching
- code of practice
- voluntary targets
- 6-week structured programme for targeted antibiotic use + biosecurity
- vaccine
- benchmarking
- regulation on sales and market authorization
- behaviour change
- regulation on antibiotic sales (prescription)
- r&d initiatives
- access initiatives
Although more general “infection, prevention and control” interventions, which we deemed AMU-sensitive for this review, have the potential for reducing drug resistant infections and antibiotic use, there is still lots unknown on the comparative and combined impacts of sensitive and specific interventions. NAPs will be implementing lots of things at once, making the quantification of specific impacts difficult using real-world evidence, though we hope to fill some of these gaps through SEFASI research. This can be seen in our hypothetical, scenario testing approach in our recently published paper “AHHME: A model for estimating the holistic cost-effectiveness of antimicrobial resistance interventions in food animal production” available here https://www.sciencedirect.com/science/article/pii/S2352771423001490.
Whilst the NAP development continues across these countries for the next round, as does research into the potential impact of interventions, it is key that we feed both of these streams into each other. In SEFASI we are trying to do this with the continued involvement of our policy and research networks and our Knowledge Hub members. Look out for the next meeting (11th January) and subsequent blogs early next year!
Table 1. A Summary of farm-level antibiotic usage objectives in SEFASI-related NAPs
| Country | Objective | Interventions | Intendant Population | Stated Measurements of Impact |
| England | 2.3. Lower burden of animal infection | hygiene-based interventions animal housing design & build group management minimum movement & mixing biosecurity freezing quality colostrum | freezing quality colostrum for calves, piglets, and lambs farmers | antibiotic use |
| England | 2.3.1 Support animal husbandry practices that prevent endemic diseases | guidance, training & communication animal health planning interventions· | farmers | antibiotic use farm economics (cost-effectiveness) |
| England | 2.3.2 Promote vaccination to prevent endemic disease | promotion of vaccine use | farmers veterinarians | |
| England | 2.3.4 Better understand how AMR spreads between and among humans, animals and the environment | gene therapies nutrition targeted interventions based on animal disease data guidance, training & communication | farmers veterinarians | |
| England | 2.5.2 Promote good practice across the food chain | Communication behaviour change code of practice | food handlers consumers food chain actors | |
| England | 3.2.1 Strengthen stewardship for responsible use | voluntary targets 6-week structured programmed for targeted antibiotic use & biosecurity | sheep; beef; dairy; poultry; egg; fish; gamebird; pig farms | |
| England | 3.2.2 Improve data and control | required electronic medicines book under key farm assurance schemes. benchmarking | pig farms
| antibiotic use |
| England | 4.5.1 Stimulate broader access to vaccines for humans and animals | promotion of vaccine use | farmers veterinarians | |
| England | 4.6. Better quality assurance of AMR health products | regulations on antibiotic sales (online and OTC) | antibiotic sellers antibiotic consumers | |
| Denmark | 5.1. 1 Monitoring the extent of all veterinarian-prescribed antibiotics. | remove market authorization for zinc targeted antibiotic use | antibiotic use | |
| Denmark | 5.1.2. Continued focus to ensure that flock medication is only applied when this is the professionally correct form of treatment. | Regulation on antibiotic sales (prescriptions) | antibiotic use | |
| Denmark | 5.1.3 Supervision of and guidance for pig veterinarians regarding prudent and reduced use of antibiotics. | guidance behaviour change | veterinarians | antibiotic use |
| Denmark | 5.1.4 Benchmarking of veterinarian’s prescription of antibiotics. | benchmarking
| veterinarians | antibiotic use |
| Denmark | 5.2.1 Prevention of direct animal-to-human transmission | hygiene interventions | farmers | |
| Denmark | 5.2 Greater efforts to prevent infections and to facilitate antibiotic alternatives | biosecurity research & development into alternatives to antibiotics | cattle; pig; poultry farms | monitoring of hygiene monitoring of AMR |
| Denmark | 5.3 Enhanced knowledge to improve targeted measures | targeted interventions based on animal disease data | resistance epidemiology knowledge | |
| Denmark | 5.4 Information and guidance on resistance and transmission | communication behaviour change
| Farmers (especially pig farmers) | knowledge & awareness |
| Senegal | 1.3 Strengthening Laboratories for AMR monitoring in the food and animal sectors | surveillance capacity targeted interventions based on animal disease data | animal sector | |
| Senegal | 3.1.1 Update national recommendations for the proper use of antimicrobials | National guidance | ||
| Senegal | 3.1.2 Update national recommendations for the proper use of antimicrobials | Training modules on rational use of antibiotics teaching; communication; behaviour change | ||
| Senegal | 3.3. Strengthen the institutional and Regulatory process | regulation on antibiotic use supporting fight against illegal sales of antibiotic to enforce regulation | Farmers Fishing industry | |
| Senegal | 3.4.1 Monitor antibiotic consumption following the WHO/OIE approach | benchmarking | antibiotic use | |
| Senegal | 4.1.5 Organize national human and animal medicine awareness campaigns against the dangers linked to the use of street drugs | communication; behaviour change | public farmers |
By Joshua Aboah and Michel Dione
Antimicrobial resistance (AMR) is a global public health threat that necessitates comprehensive strategies tailored to specific contexts. Addressing the complex drivers of AMR requires the collaborative participation of all stakeholders involved in the value chain. Globally, the poultry production sector is a high consumer of antibiotics and high levels of AMR have been found in bacteria isolated in poultry farms [1]. Yet, in Senegal, the poultry industry plays a vital role in food security and economic development. A recent article shows that there is evidence that awareness raising and biosecurity improvements could encourage prudent use of antibiotics, and that biosecurity and vaccination could to some extent replace antibiotic use as productivity-enhancing and disease management tools in broiler farms. Finally, issues of farm antimicrobial stewardship must be considered at the structural level, with farm behaviours contingent on interaction with state and private stakeholders [2].
Therefore, addressing AMR challenges by reducing antibiotic use in the poultry sector could potentially be done with little impact on production and could go a long way to supporting long term food and health security of the country. However, understanding how to combine this information to effectively combat AMR within the poultry sector is complex. System dynamics modelling has emerged as a useful tool to fill this gap. This blog post highlights how system dynamics modelling is being applied to understand and mitigate AMR in Senegal's poultry value chain.
System dynamics modelling provides a holistic and dynamic approach to understanding complex systems and their interdependencies. By integrating various factors, feedback loops, and causal relationships, system dynamics models simulate the behaviour of a system over time and identify leverage points for intervention. This modelling approach facilitates a participatory model building process, allowing stakeholders to co-conceptualise problems and highlight the various interactions that contribute to systemic issues.
As part of the SEFASI project’s research activities, a two-day workshop was organised by scientists from the International Livestock Research Institute’s epidemiologist led by Dr Michel Dione, in Senegal in October 2022. The workshop aimed to structurally co-design the model structure to capture all parts of the poultry value chain with key stakeholders. Using a group model building process, the stakeholders were guided to map the pathways for AMR, identify the unintended consequences of activities to tackle AMR by key chain actors and propose strategies to combat this challenge. This collaborative workshop provided a platform for stakeholders to come together and contribute their expertise in conceptualising and designing a model to help address AMR [3]. The output from the workshop will be the foundation for developing a quantitative model and a user-friendly web-based interface that will serve as a decision-making tool for stakeholders.

System dynamics modelling enables the simulation of the effects of interventions and policies on AMR dynamics within poultry value chains. By quantifying potential outcomes, stakeholders can make informed decisions regarding the efficacy of different intervention strategies in mitigating AMR before implementing them. This proactive approach empowers stakeholders to optimise policies and allocate resources effectively.

In conclusion, it is worth reiterating that the threat of AMR in Senegal’s poultry value chain demands a multi-faceted approach that harnesses innovative tools and strategies. By utilising the system dynamics modelling approach, stakeholders in Senegal’s poultry industry can develop targeted strategies to reduce antimicrobial use, improve farming practices, enhance veterinary services, and promote responsible antimicrobial stewardship. Moreover, by optimising policies through scenario testing and evaluation, the stakeholders are able to evaluate the trade-offs between public health, economic considerations, and food security objectives. By embracing this modelling approach with early engagement and co-design, stakeholders in Senegal’s poultry industry can work together towards sustainable practices, ensure food safety, protect public health, and safeguard the future of poultry production in Senegal.
References
[1] Nhung NT, Chansiripornchai N, Carrique-Mas JJ. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front Vet Sci. 2017 Aug 10;4:126. doi: 10.3389/fvets.2017.00126. PMID: 28848739; PMCID: PMC5554362.
[2] Emes E, Faye A, Naylor N, Belay D, Ngom B, Fall AG, Knight G, Dione M. Drivers of Antibiotic Use in Semi-Intensive Poultry Farms: Evidence from a Survey in Senegal. Antibiotics (Basel). 2023 Feb 24;12(3):460. doi: 10.3390/antibiotics12030460. PMID: 36978328; PMCID: PMC10044536.
[3] Aboah J, Ngom B, Emes E, Fall AG, Seydi M, Faye A and Dione M (2023) Mapping the effect of antimicrobial resistance in poultry production in Senegal: an integrated system dynamics and network analysis approach. Front. Vet. Sci. 10:1189109. doi: 10.3389/fvets.2023.1189109
By Gwen Knight
The first Knowledge Hub meeting of the SEFASI project took place on the 1st March 2023. In this meeting we held discussions in English and French with Knowledge Hub members on a variety of questions to inform the direction of SEFASI decision making around analysis and modelling. To do this, we had a survey that we went through in the meeting and asked members to fill in in their own time with their opinions on key questions. A big thanks to all participants and respondents!
The majority of the 36 survey respondents were based in Senegal (13) or the UK (12), reflecting two of the key settings for the SEFASI project. A similar spread of participants was seen in those attending the meeting. We had a good spread of expertise, with the majority reporting expertise in epidemiology, farming/agriculture, veterinary or microbiology with a good spread across both human and animal health in both countries. We were biased to most of the participants being from academia, but did have NGO representation in Senegal. Interestingly, 21 of the respondents said they worked in the “One Health” sector.
To inform our data analysis, the first questions asked about key One Health bacteria and antibiotics to focus on. Across Senegal and the UK, there were commonalities in that E. coli and Salmonella came up as the top mentioned bacteria, and fluoroquinolones and the beta-lactams as the top mentioned drugs. However, there was a huge range of mentioned bacteria and drugs, and it is hard to know the denominator for comparison.
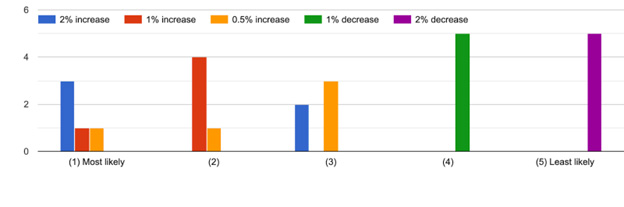
For the model scenarios, it would be key to know baseline resistance trend scenarios. During the meeting the inability to put a number on this was discussed and it came across in the free text from survey respondents. However, we had a clear result that a decrease would be unlikely (Figure 1). One of the aims of SEFASI is to raise awareness of modelling and the complexity of capturing intervention impact - we hope that questions like this, that are difficult to answer, increase the awareness of the community about the kind of assumptions that models have to make in order to evaluate interventions.
Figure 1: Knowledge Hub responses to likely future trends in resistance over the next 10 years
For interventions, we asked each country about the AMU-specific and AMU-sensitive interventions in their National Action Plans [see other blog for details of the interventions]. Here we found that none were assessed as being harmful but that “Voluntary Usage Targets” and “Surveillance data and benchmarking” or “Research and Development into alternatives to antibiotics” were dominated by “not effective” or “neutral”. Those where “very effective” or “effective” dominates were deemed to be “Infection, prevention and control”, “More targeted antibiotic use” and “behaviour change”. Interestingly, “surveillance data and targeted health interventions” was deemed “effective” whilst only benchmarking was neutral suggesting that in England, the assessment is that merely reporting data is not enough and that the data has to guide specific interventions. In Senegal, similarly none were harmful and all were seen as effective or very effective.
Finally, we asked about understanding what output measures we should evaluate intervention impact on. We had a very diverse selection here, with no clear priorities in the One Health setting in either the UK or Senegal, and a similar distribution across the two countries though the majority of the Senegal responses set “Antibiotic Residues” being key. There was a substantial focus on animal welfare and mortality as well as infection incidence in humans and antibiotic consumption. Economic indicators that ranked the highly were the monetary cost-benefit and individual income effects, whilst GDP was not often selected.
Conclusions
Next steps for Knowledge Hub development
The Knowledge Hub is currently biased towards academic partners, with some NGO connections from Senegal, and few participating members from Denmark. These biases need to be addressed to ensure we have good prior information for our parameters and analysis going forward.
We asked some potentially “unanswerable” questions (e.g. on future trends) and had this feedback from participants. In future surveys we need to ensure that we use these as learning tools: to predict intervention impact we have to know something about what happens if we don’t intervene. This could potentially be addressed by motivating examples from the modelling process.
Our experts did not clearly differentiate the interventions in terms of their effectiveness, suggesting that we have to explore all interventions from the NAPs. However, we have to reach a balance between model complexity to account for variation in interventions whilst being able to capture a range. Many of the impacts are already included in AHHME, but we don’t have an environment component so will have to work on adjusting this angle in our work.
Next steps for SEFASI
Our Knowledge Hub participants provided key inputs for moving our SEFASI research forward such as key pathogens and antibiotics to focus on, and trends in resistance. These have been integrated into the data analysis and guide our decision making as to intervention analysis.
By Ross Booton
Dr. Ross D. Booton previously worked on antimicrobial resistance (AMR) in Thailand from a One Health perspective, where human health is co-dependent on animal health and the environment. He did this with colleagues from the One Health drivers of AMR in rural Thailand (OH-DART) group.
He used a novel mathematical model framework of gut colonisation with extended-spectrum beta-lactamase (ESBL)-producing bacteria and transmission to project the reduction in human antimicrobial resistance over 20 years (2020-2040) for each One Health driver, including individual transmission rates between humans, animals and the environment, and to estimate the long-term impact of the Thai National Strategic Plan (2017) on Antimicrobial Resistance intervention.
His model predicts that human usage of antibiotics was the most important factor in reducing the colonisation of humans with resistant bacteria. The Thai National Strategic Plan on AMR was projected to reduce human colonisation by 6.0-18.8%, with more ambitious targets (30% reductions in human antibiotic usage) increasing this to 8.5-24.9%.
This model provides a simple framework to explain the mechanisms underpinning antimicrobial resistance, suggesting that future interventions targeting the simultaneous reduction of transmission and usage of antimicrobials would help to control antimicrobial resistance more effectively in Thailand.
For more information: Booton RD, et. al. One Health drivers of antibacterial resistance: Quantifying the relative impacts of human, animal and environmental use and transmission. One Health. 2021. 10.1016/j.onehlt.2021.100220.
Application to SEFASI
Ross will use this model framework to predict the potential impact of One Health interventions in England, Denmark, and Senegal. He plans to develop his model and parameterise to each of England, Denmark, and Senegal’s specific antimicrobial resistance settings. Together with collaborators in these 3 countries, he will estimate the value of intervention packages that relate to human, animal, or environmental sources. He will do this in the context of E. coli resistance to third generation cephalosporins. This theoretical modelling work could help understand which intervention would yield the greatest impact in each country and help decision makers to effectively plan national strategic action plans for AMR in the immediate future.
Recent updates
By Nichola Naylor