
Female mosquito sculpture on LSHTM building
Human Malaria Transmission Facility
Human Malaria Transmission Facility, open to the whole scientific community to study the human malaria parasites and their relationship with mosquito vectors.
Malaria remains one of the deadliest infectious diseases worldwide. Despite an overall reduction in worldwide cases and deaths since the turn of the millennium, progress in control and elimination of malaria has stalled and even reversed.
To further reduce malaria incidence, new approaches and interventions are required, alongside a collaborative effort between the global scientific community. One important, but understudied, part of the malaria lifecycle is the development of the parasite in the mosquito. To promote the study of these stages of the parasite’s lifecycle, we have established a transmission facility at LSHTM where human malaria parasites can be transmitted to mosquitoes.
This facility is open to all members of the research community. We aim to facilitate research into compounds and vaccines that can block transmission, parasite genes essential for transmission, and parasite-mosquito interactions. Our facility will open up new avenues of research and innovation to accelerate our fight against this devastating disease.
Recent updates
Events
Newsletter
Contact us
Let us know if there is malaria transmission work you would like to carry out. Fill in the form to provide further details.
Progress towards malaria control and elimination has stalled in recent years and new interventions are urgently required to support existing control measures. These control measures are under threat due to widespread insecticide and drug resistance. Moreover, treatments targeting the asexual stage of the parasite lifecycle, the stage causing malaria pathogenesis, focus on reducing morbidity at the individual level but often do not affect transmission. The mosquito-transmissible sexual stage parasites, the gametocytes, are insensitive to most antimalarials, therefore drug treatment frequently still permits the transmission of parasites to mosquitoes, thus allowing the disease to be spread.
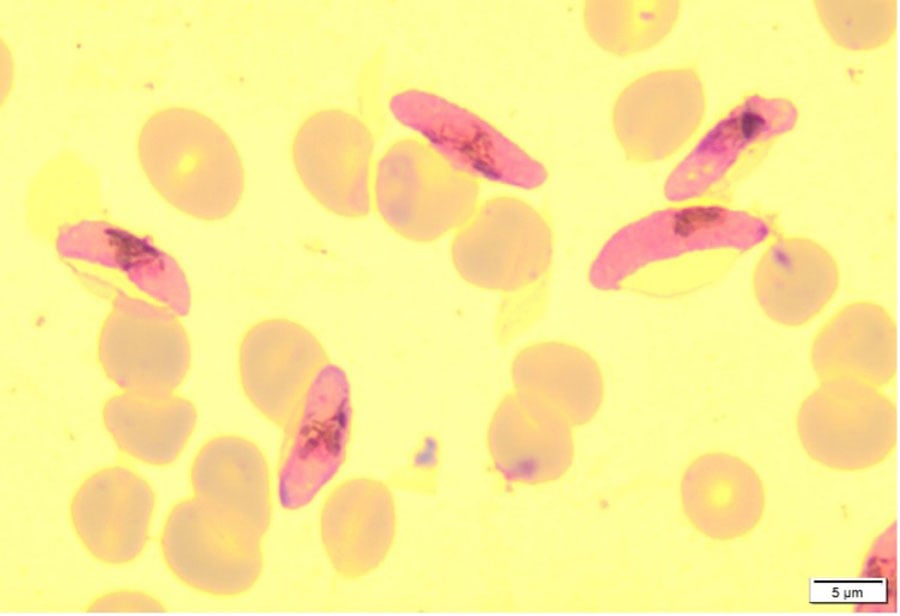
In addition, mosquito and liver stages form natural bottlenecks for parasite populations, resulting in reduced genetic complexity. For these reasons, the experimental study of parasite transmission, mosquito-parasite interactions and liver stage infection are major priorities for antimalarial drug discovery, vaccine development, basic cell biology and vector transmission research. Successful experimental malaria transmission involves understanding and managing the cell biology of three organisms – the parasite, the mosquito and the mammalian host. At LSHTM, we have combined our long-standing expertise in all three of these areas to establish a human malaria transmission facility.

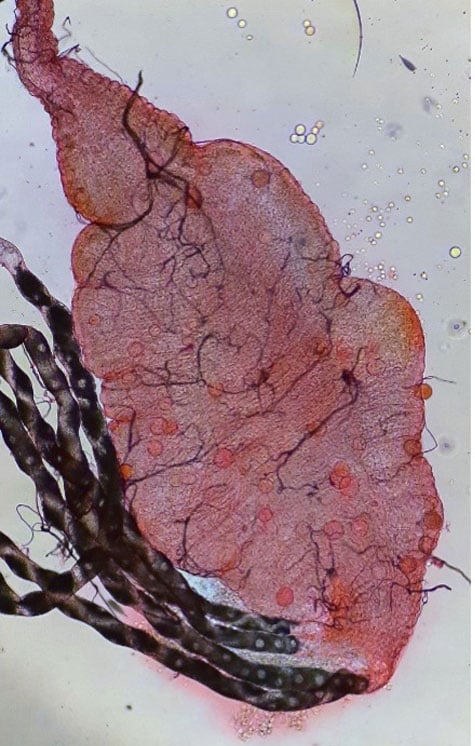
Our facility provides open access for the experimental infection of mosquitoes with P. falciparum, and other human Plasmodium species. We work with members of the research community to facilitate experimentation, drug discovery efforts and small-scale vaccine-strain testing of Anopheles mosquitoes infected with human malaria parasites.
Other than an ongoing commitment to standardising, optimising, publishing and training others in effective malaria transmission protocols, the facility’s activities are driven solely by the needs of the malaria research community. The facility is able to receive and prepare mutant parasite strains supplied by researchers for study at any stage from gametocytes to the liver stage of the humanized mouse model, test the efficacy of transmission-blocking compounds and antibodies, set up genetic crosses of parasites and supply biomaterials for multiple ‘omic analyses. This is currently done free of charge.
Recent updates
Events
Newsletter
Contact us
Let us know if there is malaria transmission work you would like to carry out. Fill in the form to provide further details.

Colin
Sutherland
Professor in Parasitology and Deputy Director for Science - MRL / Principal Investigator

Mojca
Kristan
Assistant Professor / Facility Manager and Researcher (Acting)

Harry Pollard
Higher Scientific Officer

Michael
Delves
Associate Professor / Scientific Advisory Group
Lindsay
Stewart
Higher Scientific Officer
Luke Brandner-Garrod
Research Assistant
Recent updates
Events
Newsletter
Contact us
Let us know if there is malaria transmission work you would like to carry out. Fill in the form to provide further details.
Experimental endpoints
Within the facility our team design and execute studies to interrogate different stages of the malaria transmission cycle using Plasmodium gametocytes grown in vitro in the laboratory or from clinical samples received in the UK HSA Malaria Reference Laboratory, and fed via standard membrane feeding assays to our various colonies of Anopheles mosquitoes. We then dissect mosquitoes at various time points to compare treatments groups, with a primary focus on ookinetes, oocysts and sporozoites (as shown below).

In the image above: 1) Plasmodium falciparum ookinete 28 hours post infective feed, stained using anti Pfs 25 monoclonal antibodies. 2) Plasmodium falciparum oocysts 7 days post infective feed, stained with 0.5% mercurochrome. 3) Plasmodium falciparum sporozoites 14 days post infective feed, visualised with a phase contrast microscope.
Research focus
We currently focus on four key areas of research, although we are open to expanding this depending on the research interest of potential collaborators. This includes:
- Parasite knockout lines (includes conditional KOs) and their impact on transmission
- Transmission blocking compounds / vaccines
- Effect of insecticides, endectocides and insecticide resistance mechanisms on transmission
- Drug resistant phenotypes and parasites with deletions, and their impact on transmission
Consistent transmission in 2023
Experiments in 2023 have yielded consistent Plasmodium falciparum transmission using our NF54 wild type strain.
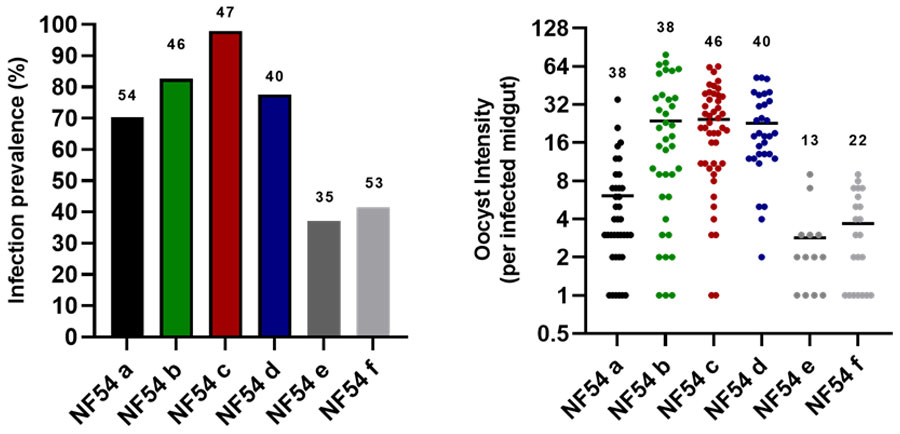
In the image above: 1) Infection prevalence of our control wild type NF54 line across feeds in 2023 using An. stephensi (SD500). 2) Oocyst intensity (per infected midgut) with our control wild type NF54 line across feeds in 2023 using An. stephensi (SD500). Y axis is represented in a log2 scale.
Recent updates
Events
Newsletter
Contact us
Let us know if there is malaria transmission work you would like to carry out. Fill in the form to provide further details.
We are located in the LSHTM Keppel Street building. Our facility encompasses three laboratories:
The insectary
We house various species/strains of Anopheles mosquitoes, including An. stephensi (SD500), and An. coluzzi (N’guosso). Depending on study requirements we are open to setting up and rearing new colonies of mosquitoes were required.
The CL3 culture lab
This is where the culture of the P. falciparum asexual and sexual stages takes place. Our main gametocyte-producing strain is NF54, though we are open to testing different strains including KO lines.
The CL3 Transmission suite
Located on the 4th floor of the Keppel Street building, this suite offers a CL3 level of security. Here we carry out the transmission experiments.
Recent updates
Events
Newsletter
Contact us
Let us know if there is malaria transmission work you would like to carry out. Fill in the form to provide further details.
Recent updates
Events
Newsletter
Contact us
Let us know if there is malaria transmission work you would like to carry out. Fill in the form to provide further details.
