In 2014, the WHO, in collaboration with the World Health Assembly, developed an End TB Strategy by 2035. The strategy focuses on reducing the incidence of TB and mortality. Targets aim to meet the Sustainable Development Goal (SDG) of ending TB by 2030 by reducing TB deaths by 90% and incidence by 80% (WHO, 2021). The targets extend to 2035 and aim to achieve a 95% reduction in mortality and a 90% reduction in incidence, as a result declaring the TB epidemic eradicated and no longer a public health problem (WHO, 2015).
The global burden of TB is evident as approximately 10.6 million people developed active TB in 2021 (Litvinjenko et al., 2023). An estimated 1.6 million deaths are directly attributed to TB, with the incidence increasing by 3.6% from 2020-2021 (Litvinjenko et al., 2023; Bagcchi, 2022). Globally, populations with pre-existing vulnerabilities are disproportionately affected by TB. For example, populations experiencing low socioeconomic status, displacement, and marginalisation are more than 25 times higher than the general population (Litvinjenko et al., 2023).
What is TB?
TB is an infectious disease caused by the bacterium Mycobacterium tuberculosis (MTB), which is transmitted via microdroplets when an infected individual coughs or sneezes and is inhaled by another vulnerable individual (NHS, 2023). MTB manifests into active TB, either pulmonary (PTB) or extrapulmonary (EPTB). PTB is where the infection which mainly involves the lung but can spread into other organs; and EPTB is an infection which involves multiple organs such as skin, bones, lymph nodes being infected (Lee, 2015). There is a discrepancy in case reporting of EPTB as it is harder to diagnose compared to PTB. EPTB accounts for 20-30% of all TB cases (Gopalaswamy et al., 2021).
Symptoms of TB are typically non-specific, such as persistent cough, weight loss, fatigue (NHS, 2023). However, delays in seeking treatment for active TB can be 30 days or more, depending on social determinants of health and how prevalent/endemic TB is in the context (Roberts et al., 2020). Additionally, TB can also lay latent, known as latent TB infection (LTBI), which is classified as remaining asymptomatic for two or more years (Lee, 2015). Approximately 28% of the global population has been exposed to MTB, but over 90% will not go on to develop active TB (Kusejko et al., 2020). However, pre-existing conditions such as chronic immunosuppression among HIV patients activate LTBI (Sharan et al., 2020).
How is TB treated?
Diagnosis encompasses many processes, beginning with a presentation of symptoms of reasonable suspicion of a case. Skin tests (cultures) are conducted to examine the presence of acid-fast bacilli; a positive test indicates the patient is likely to have LTBI or active TB (Mayo Clinic, 2023). Serological testing can be done to examine the presence of antibodies within a blood sample, and X-rays can be used to visually indicate the presence of active TB (ibid.). Testing the sputum can be done using nucleic acid amplification tests (NAATs), which detect MTB and have high sensitivity and specificity (WHO, 2021). Specifically, NAATs detect genic materials (RNA/DNA), which facilitates earlier diagnosis compared (24-48hrs) to antibody and/or antigen tests (ibid.). NAATs can detect mutations associated with drug-resistant strains of TB.
The WHO (2022) recommends conducting rapid drug susceptibility testing (DST) before beginning treatment. This is to confirm the case and if drug susceptibility/resistance is present and make necessary treatment adjustments. Treatment of drug-susceptible TB is antibiotics for at least six months of rifampicin (WHO, 2022). A longer treatment duration of 9-12 months is recommended when TB has impacted the central nervous system, bone, or joint (ibid.). However, approximately 85% of cases are successfully treated due to multiple determinants, such as treatment adherence (Qiu et al., 2022).
Supportive care is sometimes necessary when additional comorbidities such as malnutrition are present. The WHO (2022) defines malnutrition as an imbalance of essential nutrients, deficiencies or excess nutrient intake, or impaired absorption. There are three categories: obesity, overweight, and undernutrition (ibid.). Undernutrition is commonly associated with poor socioeconomic status and infections such as TB and HIV (WHO, 2013). Patients with the underlying condition of undernutrition are particularly vulnerable to TB infection. The comorbidity weakens the immune system, which increases the progression from LTBI to active TB, further weight loss, nutrient and metabolic deficiency, and malabsorption of TB antibiotics (WHO, 2013). Overall, TB-infected patients with undernutrition experience quicker disease progression, worse disease manifestation, and health outcomes. In this case, supportive care would be administering macronutrient and micronutrient supplementation (ibid.). However, in low-resourced settings with a high prevalence of malnutrition and TB, there is often insufficient capacity of the healthcare system and social security network to accommodate.
MDR-TB
Globally, there were approximately 450,000 new cases of multi-drug resistant TB (MDR-TB) in 2021, which increased by 3% from 2020 (WHO, 2022; Bagcchi, 2022). The most significant proportion of MDR-TB cases are in India (26%), Russia (8.5%), and Pakistan (7.9%), which comprise the majority of the global burden of disease (WHO, 2022).
MDR-TB is transmitted the same way as TB. Resistance occurs due to spontaneous and random chromosomal mutations, which results in reduced susceptibility to agents (Jang and Chung, 2020). In this case, the MTB are resistant to rifampicin and isoniazid (CDC, 2020). There are two pathways of resistance: primary and secondary. Primary resistance occurs when patients are exposed to and consequently infected with an already drug-resistant strain of TB, and secondary resistance develops mainly due to poor adherence to the drug regime (Jang and Chung, 2020). MDR-TB is influenced by numerous social determinants of health, such as improper clinical management and surveillance and pre-existing conditions, like those which suppress one’s immune system (HIV/AIDS) (Litvinjenko et al., 2023).
Challenges: diagnosis and treatment
One of the main challenges to controlling MDR-TB is diagnosis and treatment. According to the WHO (2022; 2023), less than 40% of patients were notified about their diagnosis, and only 2 out of 5 people diagnosed access treatment. Identifying MDR-TB-infected individuals is challenging, particularly in low-resource settings. Kuznetsov et al. (2014) found that in the region of Russia, the active case found patients adhere to treatment more (90.1%) than patients found by passive case finding (24.6%).
Conducting rapid culture testing and DST is paramount to identifying drug-resistant cases. However, in low-resourced settings, there are issues with their specificity, sensitivity, cost, and labour (Institute of Medicine US, 2012). Additionally, DST requires laboratory confirmation, and it can take 8-12 weeks for MTB to be grown in the lab, which is difficult when laboratory capacity is substandard with limited resources. As a result, the capacity to diagnose is limited, which is especially problematic in high-burden countries.
The WHO (2022) recommends a treatment regimen of a combination of four drugs (bedaquiline, pretomanid, linezolid and moxifloxacin) for six months. However, less than 10% of patients qualify, and the majority of drug treatment is 18-20 months (Lange et al., 2018). The drugs are highly toxic with side effects, and the long duration of therapy is one determinant of poor adherence and health outcomes, which contribute to the mortality and relapse rate among patients.
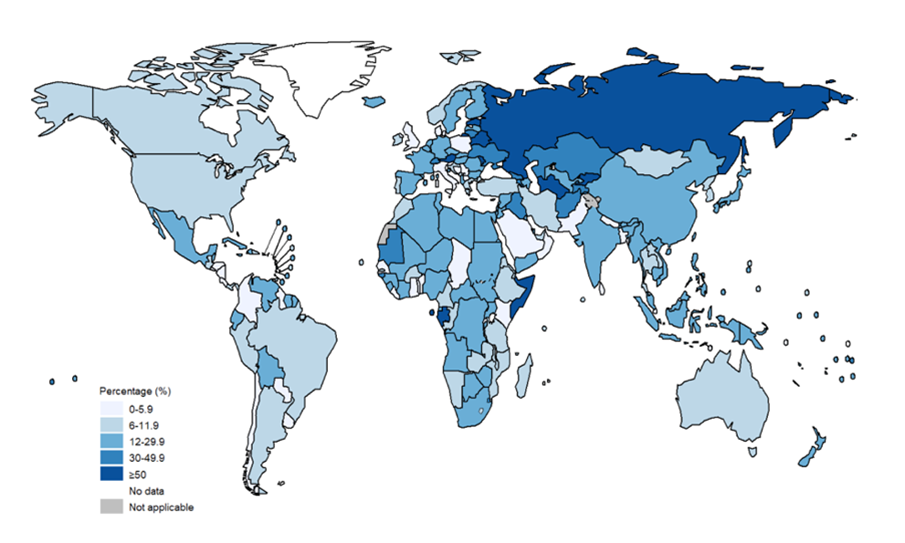
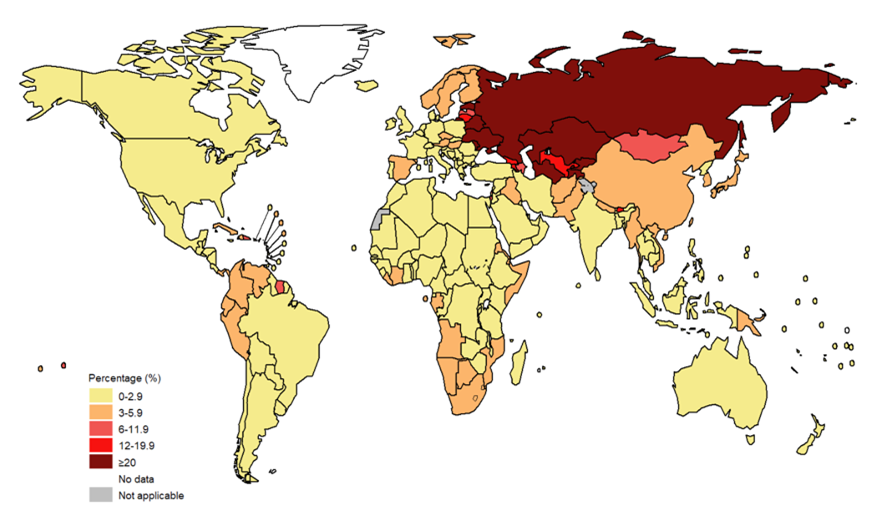
Globally, an estimated 20% of MDR-TB patients were initially diagnosed and treated for TB, compared to only 3.4% of patients who were newly diagnosed with MDR-TB (Jang and Chung, 2020) (see Figures 1 and 2). Consequently, this emphasises the limited success in treatment outcomes of approximately 54% (Qui et al., 2022). High rates among those who previously had TB may suggest that previous exposure to antibiotics may also contribute to MDR-TB infection due to high toxicity (Chung-Delgado et al., 2015). Previous exposure may indicate the patient is already predisposed to higher reinfection risk.
Intervention: personalised treatment
Currently, there are debates about whether personalised treatment would increase positive health outcomes and decrease transmission of MDR-TB compared to standardised therapy, such as the WHO’s guidelines (Prasad and Srivastava, 2013). However, guidelines are often not feasible in lower-resourced endemic countries as they require a functioning health system which has the capacity, is well-resourced, and has the ability to follow up with patients.
A patient-tailored approach would potentially increase adherence capacity. For example, the strategy is based on prior patient history, such as pre-existing conditions, previous diagnosis of TB, geographic location, and their DST test. Accounting for and monitoring determinants of health and patient preferences is paramount in creating a manageable regime for the patient (Migliori et al., 2020). A challenge in low-resourced endemic setting is integration and coordination of multiple sectors.
Patient preferences refer to a multitude of aspects of treatment and adherence. As Prasad and Srivastava (2013) highlighted, the issue with a drug regime of 6 or more drugs increases the likelihood of intolerance by the patient and consequently negatively impacts adherence and health outcomes. Compounding factors of several drugs, method of administration, frequency and duration combined with severe toxic side effects have been shown to deter patients' adherence. For example, 30-54% of patients who experienced side effects discontinued using one or two drugs from their regime (Prasad and Srivastava, 2013).
Loss to follow-up (LTFU) is defined as the interruption of treatment for over two months (Law et al., 2018). An estimated 15% of all MDR-TB patients are classified as LTFU, which is another determinant of poor adherence (ibid.). Many socioeconomic factors, such as low income, poverty, gender, institutions, and laws/regulations, influence LTFU. Indirect costs arise, such as loss of wages/income and travel, which have been linked to catastrophic spending (incurred by the patients and their families. Ghazy et al. (2022) define catastrophic costs as total costs related to TB management which exceed the annual household income of 20%. Catastrophic costs among MDR-TB patients were 81% and 81% for HIV co-infected patients compared to 32% among drug-sensitive TB patients (ibid.). These costs are a barrier to adherence to treatment and contribute to adverse health outcomes.
Adopting a personalised approach to treatment can, to an extent, reduce LTFU while simultaneously increasing adherence and halting transmission. In recent years, more studies have examined the effectiveness of home-based therapy, which has an entirely oral treatment regime. In turn, this signalled a shift towards self-administered therapy (Law et al., 2018). Horter et al. (2014) conducted a study utilising semi-structured interviews and focus-group discussions to examine patient perspectives on home-based care. Results found common beliefs that home-based treatment is conducive to recovery, facilitates psychosocial support, and allows for more free time and earning potential (ibid.). Additionally, patients expressed that this treatment method was safer as they were not exposed to hospital-based infections.
There are concerns that home-based care potentially increases the risk of transmission and adherence. Hence, additional education is necessary for patients, their families, and their contacts. Feelings of empowerment, responsibility, and control may lead to behaviour change and consequently increase patient adherence. Indirectly, outpatient care can possibly reduce the healthcare system's burden on healthcare workers in resource-constrained settings (Law et al., 2018).
Overall, patient-centred, personalised MDR-TB treatment, whether home-based or hospital-based, can increase adherence, decrease LTFU, and improve health outcomes. Furthermore, it requires a multidisciplinary approach that includes epidemiologists, healthcare workers, patients, policymakers, and more. However, more research is needed to fully conclude the effectiveness and feasibility of these approaches, particularly in resource settings, in order to be set on policymakers’ agendas and achieve the End TB 2035 targets.
Recent research at LSHTM and on the broader literature
LSHTM PhD candidate Claire Calderwood and Marcello Scopazzini have been pivotal to the formation of the article. Claire is currently completing a PhD in Zimbabwe examining household contacts of TB infected patients as a risk group for the development of TB and chronic diseases. Additionally, how an integrated approach to screening and care for TB and other chronic conditions can be used for patients and their household contacts to meet an unmet burden. Marcello is currently completing a PhD in Zambia examining the relationship between TB and cardiovascular disease, examining the burden of disease and pathology among patients living with and without HIV. Special thank you to the both of them!
Chronic TB and the debate of personal vs. standard treatment are highly topical across other chronic conditions, both communicable and noncommunicable. LSHTM’s Centre for Global Chronic Conditions has actively explored this literature and helpful sources to find out more.
At LSHTM, there are a vast number of research projects and initiatives on TB, notably the TB Centre. The centre holds a series of lectures on various TB-related topics, such as comorbidities of HIV TB coinfection and social determinants of health. Additionally, LSHTM’s Johnson and Johnson Satellite Centre for Global Health Discovery has focused on improving drug regimes for TB and MDR TB and reducing the risk of developing and transmitting MDR TB.
In January 2023, the WHO established the BPaLM (bedaquiline, pretomanid, linezolid and moxifloxacin) Accelerator Platform (WHO, 2023). BPaLM is an all-oral treatment regimen that lasts six months, which is progress towards decreasing the duration of treatment. Additionally, the WHO called for a call to action for member nations to implement this approach and increase education and surveillance.
References List
By Scarlet Barker, MSc Study of Infectious Diseases, LSHTM
Read more:
- WHO End TB Strategy by 2035
- The TB-undernutrition syndemic: Evidence from the RATIONS trial of nutritional supplementation in Jharkhand, India
- TB vaccines, diagnostics and biomarker discovery: the MRC Gambia TB case-contact platform
If you enjoyed this article and would like to build a career in global health, we offer a range of MSc programmes covering health and data, infectious and tropical diseases, population health, and public health and policy.
Available on campus or online, including flexible study that works around your work and home life, be part of a global community at the UK's no.1 public health university.