Woman researcher with pig in Cambodia. Credit: James Rudge
When UK vet Jonathan Cranston woke in the night in 2013 with severe chest pain lasting for half an hour, he was understandably concerned.
“It felt like I had been stabbed and I was struggling for breath,” he remembers.
Otherwise fit and healthy, the 32-year old managed to get back to sleep and thought no more of it until two weeks later when night sweats began and he developed a terrible cough.
His housemate at the time, a GP, diagnosed pneumonia, but antibiotics failed to shift the illness. A few weeks later, on a family trip to St Petersburg, his father, also a doctor, was so concerned by his son’s lack of energy that he referred him for a chest X-ray on their return.
Neither the medics in his family, nor the doctors who went on to treat him in hospital, could have conceived the source of his illness – a wildebeest.
Jonathan had contracted bovine tuberculosis, the animal form of this potentially fatal lung disease, in a rare example of zoonotic disease – where infections pass from animals to humans.
A domestic vet in the UK, Mr Cranston had been travelling to South Africa every year since 2011 to aid animal conservation efforts, working on a variety of projects including dehorning rhinos to prevent poaching, helping teach veterinary students, and understanding animal welfare.
Six weeks before his symptoms began, he was on one of these trips in a town just outside Kruger National Park. He had been working head to head, literally, with 40 wildebeest as they were given anaesthetic.
The animals were being anaesthetised as part of research into their stress levels when living in an enclosed environment.
He had been up close, and exposed, to the wildebeest for up to 40 hours over a two-week period.
“I had my hands in and out of their mouths, pulling their tongues around, covered in saliva,” he said. “A world expert in zoonotic TB Dr Francisco Olea-Popelka who I later met at a conference said I must have had a cut on my hand and that’s how I was exposed.”
Hospital doctors in the UK began him on treatment for human TB, but switched drugs once the results of a pleural biopsy proved he had the bovine variety.
After nine months of eight pills a day, he was finally declared TB-free.
“The doctors were very surprised,” he said. “To the extent that they wanted to write me up as a case study, as they couldn’t find a case like mine in any published literature.”
While Jonathan’s case may be unusual in the UK, zoonotic diseases on the whole account for a wide range of infections and deaths worldwide.
The World Health Organization estimates there are 200 different zoonoses, with bovine TB the fourth most serious, causing 100,000 deaths globally each year.
Zoonotic gastrointestinal disease is responsible for the most deaths globally, at one million.
An influential 2012 study by researchers from the International Livestock Research Institute, estimated that around 60% of all human diseases are zoonotic and in the least developed countries, 20% of all human sickness and death are due to zoonoses, which are diseases that have jumped species from animals to people.
An increasing source of infection
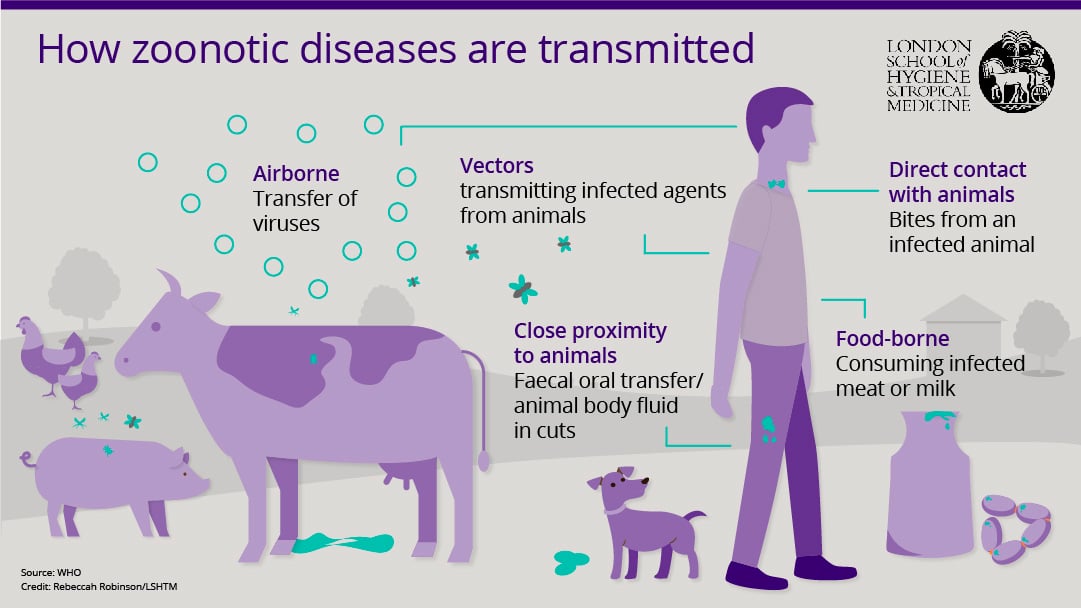
As well as TB, leading infections that transfer from animals include rabies, leptospirosis (a bacterial infection spread by cattle, pigs, dogs and rats) and sleeping sickness.
The highest rates of associated illness and death are in Ethiopia, Nigeria, Tanzania and India, with most human infections coming from livestock, including pigs, chickens, cattle, goats, sheep and camels.
There are three categories of zoonoses - endemic, which affect many people and animals in many places; epidemic, which manifest as sporadic outbreaks; and emerging, which newly appear in a population (or have existed previously but are now rapidly increasing).
It’s becoming clear that an increasing number of the new infections that have emerged in the last 10 years are of animal origin – an estimated 75% of them.
This rise has led to the formalisation of a growing global movement known as One Health, which advocates collaboration and coordination between those responsible for animal health, public health and the environment.
For example in the UK, Public Health England officials meet once a month with colleagues from the Department of Food Environment and Rural Affairs (DEFRA) and staff from leading veterinary and public health laboratories.
“They look at animal infections in the UK and elsewhere and do a risk assessment to see if any might be a threat to humans, and if so how they could be prevented, and whether guidelines are needed,” explained David Heymann, Professor of infectious Disease Epidemiology at the London School of Hygiene & Tropical Medicine and head of the Centre on Global Health Security at Chatham House, London.
In a separate initiative, the School has partnered with the Royal Veterinary College in its own project, while also jointly delivering a One Health MSc.
Osman Dar, director of the Centre on Global Health Security's One Health project, and part time consultant in international public health at Public Health England, believes “moving the preventive aspects upstream towards the animal and wildlife preserve makes sense”.
Southeast Asia is considered a hotspot where new diseases might emerge, or spread, quickly, partly because of the high densities and close proximity of people to so many animal disease “reservoirs” – such as pigs, cattle, poultry and wild birds and animals.
These animals can harbour a range of infections, including influenza in pigs, poultry, and wild birds, Japanese encephalitis in ducks and pigs, and Streptococcus in pigs.
The School’s researchers are trying to predict which agricultural and farm workers are at greatest risk and where the next outbreak or emerging illness may come from.
Calculating the risk
In Cambodia, infectious disease epidemiologist James Rudge has been exploring influenza and carrying out a study to find out which type of farming system might pose the greatest risk, firstly of flu transferring from pigs to humans, followed by its potential to become a pandemic.
Dr Rudge is Assistant Professor in the Communicable Diseases Policy Research Group and has been taking nasal and blood samples from pigs to determine which types of farms have the most flu cases among their animals, and which types have the most diverse range of flu viruses, which may be more likely to generate a pandemic.
He has also been asking swine workers to answer questionnaires on their patterns of exposure to livestock.
The study involves smallhold farmers with fewer than 10 pigs, small to medium farms with 11-200 pigs, large commercial farms with more than 200 pigs, slaughterhouses and traders.
Preliminary results show that around 11% of pigs in three provinces in the south west of Cambodia, an area of varied pig production, had been exposed to Influenza A, which indicates a risk of transfer to humans.
“We also found quite high levels of human contact with pigs, not only in the swine industry but also among rural communities where lots of households keep backyard pigs as their main source of savings,” said Dr Rudge.
As a result, he believes targeting backyard farmers might be an important strategy for reducing flu transmission from animal to human as well as human-to-human. But when resources are tight, targeting vets and slaughterhouse workers could have the most impact, he added.
“If you are going to vaccinate a certain number of pigs in the country against flu … The bigger farms are much more densely packed, so they might be good amplifiers for diseases to spread,” said Dr Rudge.
There is currently very little, if any, vaccination of pigs against influenza in Cambodia, he added, or indeed in many countries. “Vaccines are limited and can be expensive or difficult to deliver,” he said.
The growing threat of antibiotic resistance
Away from the potential for pandemics from various strains of influenza, resistance to current day antibiotics is considered by some to be a more urgent global health problem.
A major review on the global burden last year, commissioned by then UK Prime Minister David Cameron, suggested that while drug resistant infections currently kill 700,000 people a year, by 2050 this could rise to 10 million – more than the projected 8.2 million deaths from cancer.
Those working in the field fear a post antibiotic era in which common infections such as gonorrhoea could once again kill.
Without effective antimicrobials, medical procedures we take for granted in the developed world such as Caesarean sections or hip replacements, and chemotherapy treatment for cancer, become very high risk.
To stop this in its tracks, a Lancet series recently concluded that a ‘One Health’ approach to an antimicrobial resistance (AMR) strategy and policy development was vital.
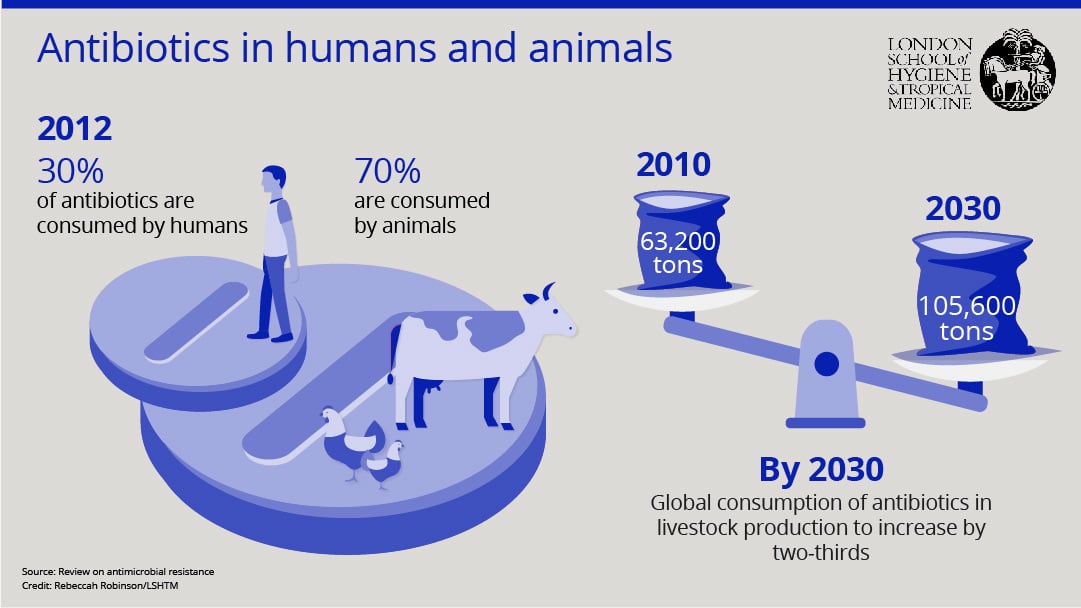
“In many countries, most antibiotics are used in the animal sector. A good statistic to highlight the scale of the issue is that 80% of antibiotic use in the US is in animals,” said Dr Dar.
“And 90% of that 80% is used, not for treatment of animals, but simply as a growth promoter,” he said.
Low-dose antibiotics are commonly given to animals to encourage growth, rather than prevention or treatment of infection, and such use has already resulted in dire consequences.
For example, resistance to the drug colistin was found in two tertiary hospitals in Guangdong and Zhejiang provinces of China where it is sometimes needed as a last line antibiotic. According to Dr Dar, this resistance almost certainly arose from the thousands of tonnes of colistin used every year as growth promoter for pig production in the country.
China has now banned the use of colistin as a feed additive for animals.
The spread of AMR – and its threat -- is being fought at the School by teams within the new Antimicrobial Resistance Centre. The Centre was launched in December 2016 by the UK’s Chief Medical Officer Professor Dame Sally Davies and brings together researchers from very different disciplinary backgrounds to collectively address this crucial public health problem.
With more than 100 members, it is split into five pillars: biological and pharmacological sciences; clinical and veterinary sciences; humanities and environmental science; epidemiology and modelling; and economic, social and political sciences.
One of the first research projects is bringing together people from microbiology, clinical sciences and social sciences to look at AMR in five different low-middle income countries - Laos, Malawi, Mozambique, Myanmar and Zimbabwe - focusing on how people use antibiotics and where resistance is emerging.
AMR among humans in developing countries is inevitably affected by AMR in animals, agrees Antimicrobial Resistance Centre co-director Clare Chandler, Associate Professor in Medical Anthropology. But it is not known what proportion of AMR is caused by overtreatment in animals, she said, meaning more research is needed.
“So if our animals at home have drug resistant microbes, we are very likely to have drug resistant microbes too.” - Dr Clare Chandler
“Our bodies are made up of 98% microbes, so of course our microbiomes – the flora in our gut, our mouth, all parts of our body - are going to change according to where we are, to the type of water we’re drinking, the type of food we’re eating, and the kind of people – and animals - that we come into contact with,” she said.
Sharing close quarters with an animal will inevitably influence a person’s microbiome, she said.
“So if our animals at home have drug resistant microbes, we are very likely to have drug resistant microbes too,” said Dr Chandler.
(Too) easy access to drugs
In rural West Bengal in India, a project led by Meenakshi Gautham, Research Fellow in Health Systems and Policy Analysis at the School and the Liver Foundation in Kolkata, demonstrates the complexities and interrelation of antibiotic use with human and animal health.
Here, people not only live with their cows, buffaloes, goats and sheep, but in some cases the same health workers that treat the humans also treat the animals.
Residents in the region are treated by village ‘doctors’ – known as informal healthcare providers (IHPs) who are not medically trained but sell antibiotics, which is technically illegal, often for use in animals as well as humans.
Initial results from Dr Gautham’s research suggest up to a quarter of IHPs may treat animals as well as people.
One IHP interviewed by Dr Gautham, who must remain anonymous, said he commonly helps the vet at the local Government hospital with animal surgery, but also travels around villages treating minor illnesses in domesticated cows, buffaloes, goats and poultry as well as conducting animal deliveries and suturing small wounds.
He learned his veterinary skills from a vet and his human health skills from formal medical doctors. He uses antibiotics to treat humans and animals, but for different durations: about 3-5 days for humans and 1-3 days for animals.
Dr Gautham and her colleagues are therefore studying why and how antibiotics are used in people by surveying a sample of 300 IHPs from two districts of West Bengal, then interviewing 30 of them in-depth.
She also has first-hand experience of their care.
While in the field in West Bengal, she fell ill with vomiting, diarrhoea and fever and had to seek treatment from one of the village doctors herself.
“He gave me three doses of an antibiotic, three paracetamol tablets, three anti-spasmodic tablets, three or four antacid tablets and a packet of ORS for 90 rupees (US$ 1.38), along with a prescription,” she said.
“I have experienced what it feels like to need treatment in an area where there are no formal medical facilities nearby, not even a pharmacy and no immediate transport to take you to the nearest facility.”
Eight hours after receiving her medication, she felt much better.
But Dr Gautham was then able to complete her antibiotics course by buying further pills in the city when she returned -- unlike the villagers she left behind.
Incomplete courses are another factor fuelling resistance, which is a problem in high income countries as well although not necessarily due to cost factors.
Typically, the IHPs only give as many doses of a pill as a person can pay for – so may only give out two or three pills - which could have two consequences: first, the number would be clinically ineffective in killing all the disease-causing bacteria and second, it would contribute to the multiplication of resistant bacteria over time.
“Would I have been able to afford the full course if I were a poor village woman? Most likely not, and I would have probably stopped with the first few doses once I felt better,” said Dr Gautham, adding that her team got direct evidence of this through their discussions with locals in the region “They said they tried to complete the dose for their children, but not for themselves as it was expensive.”
“In my view, any attempt to ban the use of antibiotics by providers who do not have a university degree in medicine would be impractical and a travesty of human rights for the large numbers of people whom they serve,” said Dr Gautham.
She believes that in low resource settings, health officials need to ensure people’s access to essential and affordable life-saving antibiotics while designing interventions to reduce the excess or inappropriate use.
Training programmes have been introduced in an attempt to improve care and reduce inappropriate use of antibiotics, but a randomised trial of a nine-month training scheme of IHPs in West Bengal using ‘mystery patients’ showed they remain averse to cutting down.
Dr Gautham’s work so far indicates the IHPs do not solely give out the drugs for financial gain, they do want to help people as well, and their main sources of knowledge are the pharmaceutical industry and formal doctors – who both promote use of antibiotics.
“They’re not trying to cheat their patients, they’re not trying to kill them, they’re actually trying to help them,” said Dr Gautham. “They are very eager for new knowledge on how best to treat people.”
The IHPs don’t usually charge a consultation fee, she further explained, and their income is based on the drugs they give out. “We have to understand that and address that issue very carefully,” she said.
Cambodia, too, faces this delicate balancing act, on the one side combating overuse, misuse, and the use of substandard products, which can result in antimicrobial resistance, and on the other enabling access to essential medicines, which can save lives.
Antibiotics. Credit: Meenakshi Gautham
Here, one of the significant players in overuse of antibiotics are ‘drug shops’ – casual, convenience stores which people go to when they are first ill, with no pharmacist or health professional.
Mishal Khan, Assistant Professor of Health Policy and Systems Research at the School, is currently working on a project to map these informal drug sellers and other providers. Previous studies by others in the field have shown that 70 to 80% of people first seek care at an informal private clinic or drug shop.
“Poor patients with low literacy levels who cannot afford to see a doctor go to drug shops and are often given antibiotics when they do not need them, or given the wrong dose, or sold poor quality drugs - all leading to AMR,” said Dr Khan.
“We are trying to understand how these drug shops might be connected to enforcement officers or village chiefs, in such a way that they could evade regulation or influence policies to restrict antibiotic sales through them.”
But there again lies an ethical boundary.
Dr Khan highlighted that if officials are not providing an alternative healthcare provider that can actually dispense drugs properly, then restricting the sale of antibiotics through informal drug sellers is actually removing access.
“It’s a very tricky situation for policy makers in these countries to handle,” she said.
Strategic policymaking
Global policies on AMR may be gaining momentum – the WHO produced a manual last year on how to develop national action plans - but they can only be followed through by governments if there is clarity on who and what is contributing to the problem in their own countries and contexts in which new policies may then work.
A team at the School is attempting to aid such progress in Pakistan by understanding which strategies to improve appropriate use on antibiotics may have some impact, led by Dr Khan and her colleague, Johanna Hanefeld, an expert in health policy and systems research at the School.
They are identifying and mapping all the people and institutions who might influence which policies and regulations are introduced – such as public and private hospitals, vets, private companies and Government agencies.
These include those that are currently ‘hidden,’ such as pharmaceutical companies and industry bodies who have an interest in the drugs being used.
Once the mapping is complete, they aim to investigate their support or opposition to a series of hypothetical policy interventions, such as restricting antibiotic use in unregulated health clinics or banning them in animal feed.
“Success to me would mean changing the paradigm from rapid response to infections that have emerged from the animal kingdom and moved to humans, to preventing them at the source.” - Professor David Heymann
Dr Khan stressed the importance of recognising that what motivates one country’s policy makers may not affect another’s. “In the UK, AMR has been framed to policy makers and hospitals as a major potential economic burden if not addressed now,” she said, adding that such framing may not work in low and middle-income countries, such as Pakistan.
In countries like Pakistan, she explained, the public hospitals are not equipped to treat drug resistant infections; meaning a child with drug resistant disease will therefore be turned away and likely die, but it was not a cost burden to the system.
Another example she gave is that policies that impact on poultry and livestock can be met with serious opposition, such as the animals’ importance to their owners in countries like Pakistan.
“Policy makers here joke that if a child is sick, the farmer will tell the mother to take him/her to a clinic, but if a chicken is sick he will go himself,” she said.
But Prof Heymann believes there is potential for a successful future with regard to the intersection of animal and human health.
“Success to me would mean changing the paradigm from rapid response to infections that have emerged from the animal kingdom and moved to humans, to preventing them at the source,” he said. “That’s a long way down the road.”
“In the meantime, the One Health platform, where all the animal and human public health people are working together is crucially important along that pathway.”
BIOS
- Dr Clare Chandler is a medical anthropologist and the co-Director of the LSHTM Antimicrobial Resistance Centre. Her work currently involves bringing together researchers from different disciplinary backgrounds to collectively address the growing burden of antimicrobial resistance.
- Prof David Heymann is Professor of infectious Disease Epidemiology at the London School of Hygiene & Tropical Medicine and head of the Centre on Global Health Security at Chatham House, London.
- Dr James Rudge is Assistant Professor of Infectious Disease Epidemiology at the London School of Hygiene & Tropical Medicine. His research focuses on the emergence and spread of infectious diseases in Southeast Asia, and the capacity of health systems to respond.
- Dr Meenakshi Gautham is a Research Fellow in health systems and policy analysis at the London School of Hygiene & Tropical Medicine. She is currently the Principal Investigator of a study on antibiotic use by informal private healthcare providers in the state of West Bengal in India.
- Dr Mishal Khan is Assistant Professor of health policy and systems research at the London School of Hygiene & Tropical Medicine. She is is a social epidemiologist with expertise in quantitative and qualitative health policy and systems research to improve infectious disease control programmes in South and Southeast Asia.