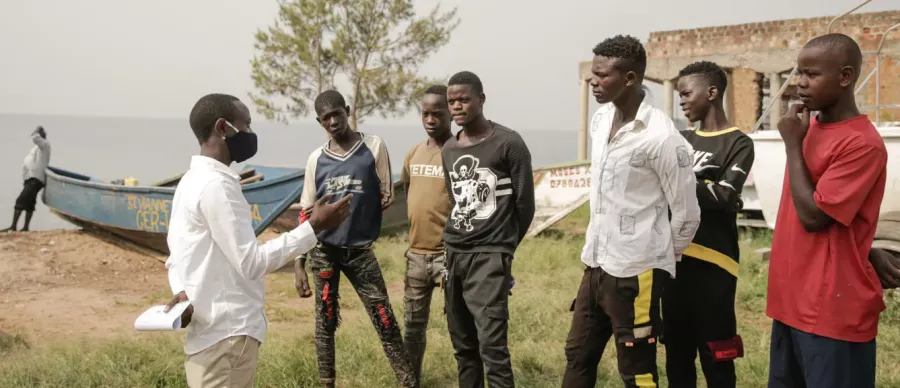
Mobile men study enagagement
As the HIV epidemic continues to pose significant public health challenges across sub-Saharan Africa, mobile men, those who frequently travel for work, remain a particularly vulnerable and underserved population. These men, including truck drivers, farm workers, fishermen and others often face significant barriers to accessing consistent healthcare, making it difficult to adhere to traditional HIV prevention methods, such as daily oral Pre-Exposure Prophylaxis (PrEP). In response to this, researchers from the MRC/UVRI and LSHTM Uganda Research Unit, in collaboration with leading institutions, have launched a study to expand HIV prevention options for this high-risk group.
Funded by the European Commission’s EDCTP Horizon program and sponsored by the London School of Hygiene and Tropical Medicine (LSHTM), the MOBILE MEN trial aims to assess the effectiveness and implementation of HIV prevention methods for mobile workers in sub-Saharan Africa. The study will compare the uptake, retention in care, coital coverage, and participant preferences between long acting injectable cabotegravir (CAB-LA) and oral Tenofovir/Emitricitabine (TDF/FTC), offered in both daily and event driven regimens. The trial, titled “Implementing Oral and Long-Acting Pre-Exposure Prophylaxis (PrEP) in Mobile Men in Sub-Saharan Africa: A Phase 3b, Open-Label, Hybrid Type 2 Implementation and Effectiveness Trial, (MOBILE MEN)” is being conducted in South Africa and Uganda.
Addressing a long-standing gap in HIV prevention
The trial comes at a crucial time, addressing a significant gap in HIV prevention for high-risk mobile populations. In South Africa, HIV prevalence among mobile workers is alarmingly high, with 26% among truck drivers and 32% among farm workers affected. Similarly in Uganda, HIV prevalence is 40% among men in fishing communities, a stark reminder of the urgent need for innovative, targeted prevention strategies particularly for populations with limited access to consistent healthcare.
The study will focus on providing simplified and community-centered PrEP delivery options tailored specifically to mobile men. This population often faces difficulties maintaining adherence to daily oral PrEP due to their constant movement and unpredictable work schedules, making it essential to offer flexible prevention methods that suit their lifestyles.
"The MOBILE MEN trial is a game-changer for HIV prevention among high-risk mobile workers in Africa. By offering flexible prevention options tailored to the realities of their lives, we are empowering these men to take control of their health in a way that suits their unique circumstances. This study has the potential to not only reduce HIV incidence but also inform future healthcare strategies for underserved populations," said the Chief Investigator, Assoc. Professor Eugene Ruzagira.
Testing a flexible and personalized prevention option
400 HIV-negative men in Southern and Eastern Africa will participate in the MOBILE MEN trial. They will be randomized to receive one of two HIV prevention methods: daily or event-driven oral PrEP (Tenofovir disoproxil fumarate/emtricitabine - TDF-FTC) or long-acting injectable PrEP (cabotegravir - CAB-LA). Participants will initially be assigned one method for nine months, after which they can switch to the prevention method of their choice.
This flexible approach is designed to accommodate the dynamic lives of mobile workers, allowing them to adapt their HIV prevention method based on their work demands and travel schedules. By providing both oral and injectable options, the trial seeks to empower participants to maintain HIV protection on their terms, without the stigma that often accompanies HIV prevention in more traditional healthcare settings.
A collaborative and multinational effort
The MOBILE MEN trial is part of a larger consortium involving leading research institutions such as the Uganda Ministry of Health, the Desmond Tutu Health Foundation, the Africa Health Research Institute, Wits Health Consortium (PTY) LTD, University College London, Kings College London andAssistance Publique - Hôpitaux de Paris (APHP). This multinational collaboration ensures that the study’s findings will have far-reaching implications, contributing not only to HIV prevention efforts in Sub-Saharan Africa but also to global health strategies.
The results of the trial will offer critical insights into the most effective HIV prevention methods for high-risk mobile workers and provide recommendations for integrating these options into existing health systems. Additionally, the study will propose a cost-effective model for sustaining HIV prevention strategies for this vulnerable population.
Shaping the future of HIV prevention
Ultimately, the MOBILE MEN trial has the potential to reshape HIV prevention for mobile workers and other high-risk populations across Africa. By focusing on flexibility, choice, and community-centered approaches, the study will contribute valuable data that can inform public health policies and strategies aimed at reducing HIV transmission in regions with limited healthcare access.
To learn more about how the MOBILE MEN trial fits into the MRC/UVRI and LSHTM Uganda Research Unit’s broader efforts to control HIV and other infectious diseases, visit our Viral Pathogens research theme page.
About the key scientists:
Associate Professor Eugene Ruzagira (Chief Investigator) is an epidemiologist with extensive experience as an investigator on HIV vaccine and non-vaccine research studies. Eugene leads HIV epidemiology studies at the MRC/UVRI and LSHTM Uganda Research Unit, and is also the acting Head of its Clinical Trials Platform. He is the Principal Investigator on a number of landmark multi-site studies, working with collaborators across Africa and beyond.
Dr. Sylvia Kusemererwa (Co- Investigator) is a research scientist at the MRC/UVRI and LSHTM Uganda Research Unit. She is a Co-Principal Investigator on the Mobile Men trial in Uganda and has contributed to various HIV prevention studies: Partner’s PrEP trial for serodiscordant couples, Dapivirine Vaginal Ring trials for high-risk women and an HIV vaccine trial comparing two forms of oral PrEP, Truvada and Descovy, among high-risk men and women.
Professor Julie Fox (Project Manager) is qualified from the University of Manchester and trained in Genitourinary Medicine at Imperial College London. Julie leads the HIV clinical trials unit at Guys and St Thomas’ NHS Trust and is a Professor in HIV Infection in the School of Immunology & Microbial Sciences at King's. Julie is also Chief Investigator on national and international multi-centre intervention studies collaborating with NHS/academic partners across the UK, Europe and Sub-Saharan Africa.
About the Collaborating Institutions:
The Medical Research Council/Uganda Virus Institute/ London School of Hygiene & Tropical Medicine Uganda Research Unit (MRC/UVRI & LSHTM) is an internationally recognized centre of excellence for research and training. Its mission is to conduct high-quality research that adds knowledge and leads to improved control of infectious and non-communicable diseases in Uganda, Africa and globally, through translation of scientific findings into policy and practice, and rigorous research capacity building.
Ministry of Health - Uganda is a government body set up with the mandate of stewardship and leadership of the health sector. The Ministry of Health is responsible for policy review and development, supervision of health sector activities, formulation and dialogue with health development partners, strategic planning, setting standards and quality assurance, resource mobilization, advising other Ministries, departments and agencies on health-related matters, and ensuring quality, health equity, and fairness in contribution towards the cost of health care.
Kings College London is an internationally renowned university delivering exceptional education and world-leading research. We are dedicated to driving positive and sustainable change in society and realising our vision of making the world a better place. Through our commitment to exceptional education, impactful research and genuine service to society, we are creating positive change in our communities, both in London and on the world stage.
University College London is London's leading multidisciplinary university, with more than 16,000 staff and 50,000 students from over 150 different countries. We are a diverse community with the freedom and courage to challenge, to question and to think differently.Through a progressive approach to teaching and research, our world leading academics, curious students and outstanding staff continually pursue excellence, break boundaries and make an impact on real world problems.
The Desmond Tutu Health Foundation (DTHF) is a registered non-profit company established in association with the Desmond Tutu HIV Centre, an accredited research centre within the Faculty of Health Sciences at the University of Cape Town (UCT).Bridging rigorous academic research with community development programmes, the DTHF collaborates to find innovative solutions in the prevention and treatment of HIV, TB and related infections. DHTF is well connected international research networks, making it an influential stakeholder in the global HIV arena.
Africa Health Research Institute (AHRI) is an independent, transdisciplinary scientific research institute based across two campuses in the province of KwaZulu-Natal (KZN) in South Africa. AHRI’s research combines population, basic and translational, social, and clinical sciences to understand and intervene in the health and well-being of South African communities.AHRI works in partnership with local communities and South African academic, governmental, and other policy stakeholders and collaborates with over 60 institutions globally. AHRI prioritises the training of the next generation of African scientists.
ViiV Healthcare is a specialist pharmaceutical company born from partnerships between GSK, Pfizer, and Shionogi and is 100% dedicated to HIV treatment and research. With a portfolio of 17 HIV medicines and the world’s only HIV-dedicated research facility, ViiV is committed to pushing boundaries in HIV care and driving innovative treatments. Beyond R&D, ViiV prioritizes access to medicines through global partnerships and initiatives like voluntary licensing for pediatric formulations. Through community-driven Positive Action programmes, ViiV strengthens local healthcare services and works to ensure no person living with HIV is left behind in the global fight to end the epidemic.
The Wits Health Consortium (WHC) is a private company wholly owned by the University of the Witwatersrand, Johannesburg, primarily serving the Faculty of Health Sciences by managing contract research, sponsored activities, and services such as clinical work. Operating as tax-exempt under South African law, WHC oversees over 50 research divisions linked to academic departments, providing governance, legal, human resource, and financial management. Its board ensures proper internal control and risk management through sub-committees. WHC, with over 20 years of experience, manages around 22% of the University's total income, handling sponsor-funded projects worth over USD 72 million annually from major global agencies and donors like USAID, NIH, and the Bill and Melinda Gates Foundation.
Assistance Publique - Hôpitaux de Paris (APHP) is a globally recognized university hospital network with 38 hospitals providing comprehensive medical and surgical care to over 8 million patients annually, across all ages, for consultations, emergencies, hospitalizations, and home care. Offering 24/7 public health services, APHP treats all patients regardless of income, ensuring equitable access to top-tier care. Each year, over 1.4 million people are seen in its 25 emergency departments, and the network plays a critical role in screening, prevention, and supporting disadvantaged patients. APHP specializes in high-level treatments, including for rare and costly conditions like transplants and burn care.
For more information about the study contact the Principal Investigator at:
Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit
Plot 51-59 Nakiwogo Road, P. O Box 49, Entebbe, Uganda
+256 (0) 312 407000, +256 (0) 417 704101
Email: press@mrcuganda.org
If you enjoyed this article and would like to build a career in global health, we offer a range of MSc programmes covering health and data, infectious and tropical diseases, population health, and public health and policy.
Available on campus or online, including flexible study that works around your work and home life, be part of a global community at the UK's no.1 public health university.
