
Blood-samples. Credit: Antonio Mendes
Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE)
FIEBRE aims to reveal leading causes of fever in sub-Saharan Africa and southeast Asia. What are the main infections causing fever in children and adults, and how should they be treated?
Rapid diagnostic testing for malaria has revealed that most febrile patients in Africa and Asia do not have malaria. FIEBRE will find out what they have and how to treat them.
Funded by the UK Foreign, Commonwealth and Development Office, FIEBRE collaborators include LSHTM, Liverpool School of Tropical Medicine, Universities of Barcelona, Oxford and Otago, and partners in Laos, Malawi, Mozambique and Zimbabwe.
Study protocols, standard operating procedures, data collection tools and related materials will be made available as they are finalised and approved.
Recent updates
Potential partners are invited to submit a proposal for the utilisation of research samples obtained during the FIEBRE study.
Events
Newsletter
Contact us
London School of Hygiene & Tropical Medicine
Keppel Street, WC1E 7HT

The objective of FIEBRE is to provide evidence:
- on the most common infectious causes of fever;
- on antibiotic susceptibility of bacterial causes;
- on how local perceptions of fever affect treatment practices including the use of diagnostics and antimicrobial drugs;
- to inform clinical guidelines and algorithms on how to manage non-malarial fevers.
The FIEBRE study will help to fill the gaps in evidence by means of a multi-centre study in countries with a high burden of infectious disease from which few or no data are available. The clinical and laboratory components of the study will focus on detecting infections that are treatable and/or preventable. Ethnographic work with community members, prescribers and public health workers will seek to understand how fever is understood by different communities of practice, and how this affects treatment practices.
The results will help to inform updated, evidence-based algorithms for the management of febrile illness, and provide data that may be used to design new diagnostics and rational approaches to disease surveillance. These outputs will ultimately help health systems and providers to provide more appropriate care to patients and lead to better clinical outcomes.
Read the FIEBRE brochure (pdf) to find out what has been achieved.
Social science
We currently know little about the antibiotics being used to treat febrile illnesses and how we could safely reduce antimicrobial use. Social science research has been called for to understand our relationships with antibiotics and how we can optimise their use in fever case management. The social science component of FIEBRE seeks to understand how fever is understood by different communities of practice and how this affects treatment practices. It aims to explore how fever case management and antimicrobial use are entwined with social, economic and political life in Malawi, Myanmar and Zimbabwe.
The work includes participant observation and interviews in a variety of settings including clinics, hospitals, pharmacies, markets and households, as well as a medicines survey to identify broader patterns of antibiotic use and access at the community level. Findings will be used alongside clinical data to inform revised guidelines for fever case management that are sensitive to the contexts in which they are used. See the protocol (pdf) for more details.
What are we looking for?
Some diagnostic tests are performed at or near the point of care at each study site. Further pathogen-based diagnostic tests and quality control tests will be performed at internationally recognised reference laboratories. For more details see the FIEBRE protocol paper.
| Diagnostic tests performed at/or near point of care |
|---|
| microscopy and rapid diagnostic test for malaria (Plasmodium species) |
| HIV rapid diagnostic test/s (African sites) |
| blood culture and antimicrobial susceptibility testing |
| urine dipstick and culture (small children and older patients with urinary tract infection symptoms) |
| mycobacterial blood culture (HIV infected African adults) |
| urinary lipoarabinomannan rapid test (uLAM) to detect Mycobacteria tuberculosis (HIV infected African adults) |
| cryptococcal antigen lateral flow assay |
| Infection | Reference laboratory for testing or quality control |
|---|---|
| arboviral infection: chikungunya, dengue, Japanese encephalitis, o’nyong nyong, Zika | ELISA, plaque reduction and neutralisation test; Unité des virus émergents, L'Institut de Recherche pour le Développement, France |
| bacterial/fungal bloodstream or urinary tract infection | MALDI-TOF; Southern Community Laboratories and University of Otago (national laboratory), New Zealand |
| bloodstream Mycobacteria infection | subculture, line probe assay or sequencing, & susceptibility testing; Leibniz-Centre for Medicine and Life Sciences, National Reference Center for Mycobacteria, Germany |
| brucellosis | microagglutination test (MAT); National Brucellosis Reference Unit, Liverpool clinical laboratories, UK |
| histoplasmosis | serum Histoplasma antigen detection; MiraVista Diagnostics, US |
| leptospirosis | microagglutination test (MAT); Institut Pasteur, France |
| malaria and other blood parasites | microscopy of blood smear; Liverpool School of Tropical Medicine, UK |
| respiratory viruses: influenza and respiratory syncytial virus, other | Luminex nucleic acid amplification test; Micropathology Ltd, UK |
| rickettsial infection: Q fever (Coxiella burnetii), scrub typhus, spotted fever group rickettsioses, typhus group rickettsioses |
immunofluorescence antibody test and PCR; Mahidol-Oxford Tropical Medicine Research Unit, Thailand; and Australian Rickettsial Reference Laboratory |

Meet the team
| LSHTM | Partner teams | Social science team |
|---|---|---|
|
Prof David Mabey, Principal Investigator |
Prof Quique Bassat, ISGlobal, Principal Investigator |
Prof Clare Chandler, Co-investigator, Social Science Lead |
For more details about each country team and the organisations involved see the Where we work section.
Watch our team in action
Find out what is really involved in FIEBRE by watching our team members explain their work.
- Prof Katharina Kranzer, Principal Investigator, Zimbabwe
- Dr Justin Dixon, Social Science Co-ordinator
- Dr Shunmay Yeung, Paediatric Lead
- Prof Quique Bassat, ISGlobal, Principal Investigator
- Dr Vilayouth Phimolsarnnousith, LOMWRU
- Dr Pio Vitorino, CISM
- Dr Wamaka Msopole, Malawi
- Molly Sibanda, Zimbabwe
- Prof Clare Chandler, Social Science Lead
- Yuzana Khine Zaw, PhD Student, Myanmar
- Dr Mayfong Mayxay, Principal Investigator, Laos
- Joseph Chipanga, BRTI, Database Administrator
- Mabvuto Chimenya, Lead nurse, MLW
- Dr Somvai Singha, LOMWRU
- Felina Mhangami, Zimbabwe
- Laos team - summary of activities
Find out more about our researchers
Study governance
An External Advisory Committee (EAC) has been established to provide scientific oversight of the FIEBRE study. The members of the EAC are:
- Prof Chris Whitty (Chair), Chief Medical Officer for England & Professor of Public and International Health, London School of Hygiene & Tropical Medicine, UK
- Dr David Meya, Associate Professor, College of Health Sciences, Makerere University, Uganda
- Dr T Jacob John, Professor Emeritus, Christian Medical College, (CMC) Vellore, India
- Dr Amanda Walsh, Senior Scientist, Emerging Infections and Zoonoses, National Infection Service, Public Health England, UK
FIEBRE is funded by the UK Foreign, Commonwealth and Development Office. It is a multi-centre study conducted by the LSHTM, Liverpool School of Tropical Medicine, Barcelona Institute for Global Health (ISGlobal), Universities of Oxford and Otago, and partner institutions in Lao PDR, Malawi, Mozambique and Zimbabwe, and collaborating reference laboratories.
Partners
- ISGlobal – Barcelona Institute of Global Health
- Liverpool School of Tropical Medicine (LSTM)
- University of Otago, New Zealand
- University of Oxford
Country partners
Laos
Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit (LOMWRU)
The Mahidol Oxford Tropical Medicine Research Unit (MORU) develops effective and practical means of diagnosing and treating malaria and other neglected diseases such as melioidosis, typhus, TB and leptospirosis. MORU was established in 1979 as a research collaboration between Mahidol University (Thailand), Oxford University (UK) and the Wellcome Trust UK. It is a network of a diversity of subunits including the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit, Lao PDR (LOMWRU). This is a small clinical tropical medicine research group based at Mahosot Hospital, Vientiane. LOMWRU builds diagnostic, clinical and research capacity to help improve global, regional and Lao public health. LOMWRU’s main areas of research interest are in the diagnosis, epidemiology and treatment of malaria, rickettsial infections, leptospirosis, melioidosis, community-acquired septicaemia, central nervous system infections, the causes of acute fevers and public health aspects of medicine quality problems.
The main study site is Vientiane provincial hospital, a 100-bed hospital approximately 70 km from Mahosot Hospital. The team consists three study doctors and two laboratory technicians. The principal investigator is Prof Mayfong Mayxay with Dr Elizabeth Ashley and Prof Paul Newton as co-investigators.
Malawi
Malawi-Liverpool Wellcome Trust Research Programme (MLW)
The Malawi site is in Chikwawa district in the southern region of Malawi. Chikwawa district is 5,000 km2 with a population of 350,000 and is served by Chikwawa District Hospital. The study is taking place in Chikwawa District Hospital and a second site at St Montfort Hospital, Ngabu 30 km away has been opened up to help increase inpatient recruitment.
The work is based out of the Malawi-Liverpool Wellcome Trust Clinical Research Programme (MLW) . Established in 1995, MLW is an internationally-recognised health research institution led by Malawian and international scientists with the aim of improving the health of people in sub-Saharan Africa. MLW is built around laboratories, located at Queen Elizabeth Central Hospital, in Blantyre.
The principal investigator is Prof Nicholas Feasey and the study doctor is Dr Ed Green.
Mozambique
Centro de Investigacao em Saude de Manhica (CISM)
In Mozambique, FIEBRE is coordinated by the Centro de Investigação em Saúde da Manhiça (CISM) in collaboration with the Barcelona Institute of Global Health (IS Global).
CISM was established in 1996 with the objective of conducting biomedical research in those diseases that affect the most poor and vulnerable. The Centre includes a fully equipped laboratory including parasitology, haematology, biochemistry, microbiology, (including biosafety level III premises), molecular biology (including PCR and RT-PCR) and immunology. CISM has been running a Demographic Surveillance System (DSS) since 1996, covering the whole district’s population and it set up a morbidity surveillance system at Manhiça District Hospital (MDH) in 1998. Overall, data on over 70,000 paediatric admissions and more than 1.2 million outpatient visits have been collected in the past 18 years. CISM’s other activities include: malaria screening, microbiological surveillance; pneumonia surveillance and conducting studies on issues with an important impact on public health policies in the country.
The study is being conducted in the district of Manhiça (population 182,000 inhabitants, 2300 km2), a rural area located 90 km away from the capital Maputo. MDH acts as the referral health facility for the area. The study is also working at the Hospital Geral José Macamo in Maputo.
The study’s Principal Investigator at the site is Professor Quique Bassat, supported by co-investigators Dr Marta Valente and Dr Pio Vitorino, in addition to a larger team of Mozambican-based staff: Dr Nelson Tembe; Dr Sozinho Acácio; Dr Ajanovic Andelic, Campos Mucasse (Project manager); Vânia Afuale (Project assistant); Humberto Mucasse (Field coordinator); Teodimiro Matsena (Data manager); Anelsio Cossa (Laboratory coordinator); Manuel Muamede (Adult nurse coordinator); and Ilídio Cherinda (Paediatric nurse coordinator).
Zimbabwe
Biomedical Research and Training Institute (BRTI)
The researchers in Zimbabwe are based at the Biomedical Research and Training Institute (BRTI) in Harare. Established in 1995, the BRTI provides effective and professional research facilities including laboratory facilities for molecular diagnostics, micro-biology, serology, TB and immunology. BRTI aims to improve health and quality of life in Africa through conducting research and training. Its role is to provide the infrastructural support that researchers in all aspects of health need to become effective in influencing policy.
The study site incorporates major hospitals in Harare (urban setting) Harare Central Hospital (HCH) and Chitungwiza Hospital. These hospitals have both inpatient and outpatients care of all age groups with patients referred from local clinics and provincial hospitals. The hospitals serve urban and peri-urban communities in southern Harare. In addition, outpatients are being recruited from Glen View and Rutsanana polyclinics in south-western Harare.
The BRTI team is led by Dr Katharina Kranzer (Principal investigator) with Professor Rashida Ferrand (Co-principal investigator) and comprises: Dr Ioana Olaru (Study coordinator), Ethel Dauya (Field manager), Tsitsi Bandason (Data manager), Salome Manyau (Social science lead), Beauty Makamure (Laboratory manager) and Tendai Muchena (Administrator). Partners include the Department of Medicine and Paediatrics at Harare Hospital and Chitungwiza Hospital, the University of Zimbabwe and Harare City Health Services.
Potential partners are invited to submit a proposal for the utilisation of research samples obtained during the FIEBRE study.
The study has yielded a collection of research samples from over 10,000 participants (adults and children >2 months old), including both inpatient and outpatient fever cases as well as age- and location-matched controls. The archive consists of various specimen types for both cases and controls, which are being stored for a limited time period at LSHTM: Serum (d0 and d28), venous blood in PAXgene tubes (Laos, Malawi and Mozambique in a subset of participants), whole blood and cell pellets. Plasma may also be available (TBC). Availability per participant varies.
We wish to identify partners who are seeking well-characterised samples from a diverse study population for translational research (such as evaluations of in-vitro diagnostics or novel biomarkers) with global public health objectives aligned with that of the FIEBRE study. FIEBRE samples are not commercially available, but invited proposals for their use are those with existing funding or a clear route to potential funding for the proposed research, as well as for the management, processing and shipment (where applicable) of the requested samples. Applicants with existing or proposed capacity and/or funding for future storage and responsible management of the sample archive would be particularly desirable.
Please see the full Request for Proposal (pdf) for further details.
To submit a proposal for the use of archived FIEBRE samples, please complete this short online form with your details and declarations of interests and send a 1-2 page proposal to fiebre@lshtm.ac.uk by 4 November 2023 23:59 UTC.

Find out more about the people working on FIEBRE from around the world. The consortium is made up of an international, multi-disciplinary team including PhD students, clinicians, nurses, statisticians, anthropologists, database administrators, lab technicians and many more.
Read the profiles of some of our researchers.

The final FIEBRE co-investigators meeting took place at LSHTM from 1-3 February 2023. Over 35 representatives from our partners and collaborators around the world attended online and in-person. The agenda covered:
- group review of data available and preliminary analysis for primary and secondary objectives
- agreement on manuscript list, key messages and content of manuscripts
- agreement on next steps for further analysis and any future proposals.
There were productive discussions around quantitative results addressing primary objectives 1 and 2 (see protocol paper), results of incidence estimate analyses and microbiology results and implications. The social science team presented an overview of their findings, implications, and discussion of how quantitative and qualitative results may be integrated to inform fever management and other goals.
The study started in 2017, with the first patient enrolled in Zimbabwe in 2018. An immense amount of data from the sites in Laos, Malawi, Mozambique and Zimbabwe has been collected, collated and analysed. The data will provide some of the first estimates of disease burden for our sites.

Photos (left to right): Patient enrolment in Zimbabwe; control visit in Laos; enrolment of child in Mozambique; nurses preparing in Malawi
The American Society of Tropical Medicine and Hygiene 2022 Annual Meeting (ASTMH 2022) took place 30 October to 3 November 2022 in Seattle, USA, in person and online. There are three FIEBRE-related presentations:
- Etiology of febrile illnesses associated with seizures in children from low- and middle-income countries: results from the FIEBRE study (pdf)
Sara Ajanovic, Sham Lal, Polycarp Mogeni, Justina Bramugy, Marta Valente, Ioana Olaru, Manophab Lungraj, Vilayouth Phimolsarnnousith, Vilada Chansamouth, Mabvuto Chimenya, Edward Green, Nicholas Feasey, Audrey Dubot-Pérès,, Mayfong Mayxay, Elizabeth Ashley, Paul Newton, Katharina Kranzer, Shunmay Yeung, Heidi Hopkins, David Mabey, Quique Bassat
Scientific Session 40: Clinical Tropical Medicine: Diagnostics and Febrile Syndromes
31 Oct 2022 5.15 – 7.00 pm
-
Machine Learning-Based Prediction of Clinical Outcomes for Children using linked electronic health records in Manhiça District, Mozambique
Sham Lal, Marta Valente , Oliver Baerenbold, Justina Bramugy, Sara Ajanovic, Teodimiro Matsena, Chrissy h. Roberts, Quique Bassat - Leptospirosis among patients presenting with fever in the multi-center Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE) study (pdf)
John A. Crump, Polycarp Mogeni, Paul N. Newton, Amandine Houvert, Vallier Sordoillet, Sham Lal, Mayfong Mayxay, Edward Green, Marta Valente, Justina M. Bramugy, Chrissy Roberts, David C. W. Mabey, Heidi Hopkins, Mathieu Picardeau, on behalf of the Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE) Consortium
Late-breaker Session 64: Late-breakers in Clinical and Applied Sciences
1 Nov 2022 12.15 – 1.30 pm

BMC Infectious Diseases is calling for submissions to their BMC Infectious Diseases Series: Febrile illness – diagnosing and caring for preventable and treatable infectious causes of fever, a collection of original research articles and systematic reviews characterising the clinical, diagnostic, management, epidemiological, anthropological, and public health aspects of febrile illness in LMIC settings.
The FIEBRE team have been working with BMC to help set this up. Submission deadline: 31 Dec 2022.

Leptospirosis is a zoonotic bacterial disease. There’s limited information on the global burden of this neglected and emerging disease. The World Health Organization (WHO) estimates one million leptospirosis cases and 60,000 deaths worldwide each year, most commonly occurring in tropical and subtropical areas with high rainfall. Leptospirosis is underdiagnosed as it can cause a wide range of symptoms, for example fever, which may be mistaken for other diseases such as dengue and malaria. There’s a lack of adequate diagnostics and no effective control measures.
To establish the incidence of leptospirosis at our sites the National Reference Centre at the Institut Pasteur is testing FIEBRE plasma and serum samples. To find out more about leptospirosis and the processes involved in testing the FIEBRE samples see this presentation (pdf) provided by the Department of Microbiology team at Institut Pasteur.
Bloodstream infection is a major public health burden worldwide, resulting in significant patient morbidity and mortality. Many different types of microorganisms can cause bloodstream infections, and the causes vary widely across the world.
Bacterial and fungal infections can cause fever. Blood cultures are performed on all study patients. Urine cultures are performed for patients under 2 years of age and for older patients with symptoms of urinary tract infection. Blood and urine cultures are performed at the study sites. Laboratory staff at the study sites identify bacteria or fungi isolated from positive cultures, perform antimicrobial susceptibility testing (for bacteria), and report the results to the patient’s clinical team to assist with clinical management. For external quality assurance, each isolate is then shipped to Southern Community Laboratories (SCL), Dunedin, New Zealand. SCL is an IANZ-accredited diagnostic laboratory that provides pathology testing services to hospital and community providers in the South Island of New Zealand.
Quality assurance testing procedure at SCL:
- Isolates are identified by matrix assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-ToF-MS) and/or 16s rRNA gene sequencing.
- Antimicrobial susceptibility testing is performed and interpreted as per the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
- Further phenotypic susceptibility testing methods to detect epidemiologically important mechanisms of resistance (eg production of extended spectrum beta-lactamases or carbapenemases).
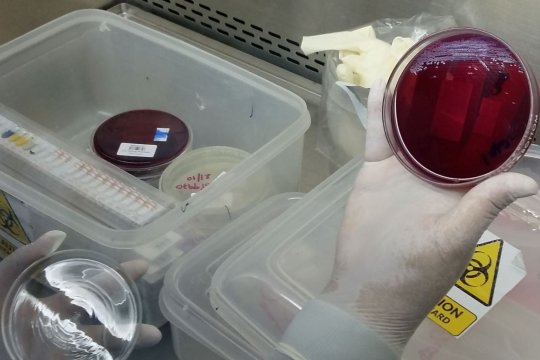
API test strip and blood culture plate in LOMWRU laboratory, Laos
Histoplasmosis is a serious fungal infection. Mild forms of histoplasmosis cause no signs or symptoms; but severe infections can be life-threatening particularly for those with weaker immune systems. It is a major cause of morbidity and mortality for people living with HIV. There’s little reliable surveillance data worldwide, especially in low-resource settings, therefore it is difficult to estimate its true burden and geographic distribution.
Serum samples from FIEBRE participants are shipped to MiraVista Diagnostics. Serum samples received at MiraVista Diagnostics are inventoried and stored at -80°C. MiraVista Diagnostics performs antigen detection by enzyme immunoassay (EIA) to determine the quantitative concentration of Histoplasma antigen in the serum samples. Prior to testing, samples are thawed, treated with EDTA and aliquoted into tubes to be tested in the MVista® Histoplasma Antigen EIA using Tecan liquid handlers, in 96 well plates. At the completion of each assay controls are verified and calculations performed. Following testing results are provided to the study coordinator for evaluation.
The frequency and scale of arboviral epidemics and the extent of their geographic spread have progressively increased over time becoming a major public health concern in the world. Infections by arboviruses clinically manifest in a range of presentations from asymptomatic infections to fever with more or less severe symptoms which need to be identified and treated correctly.
To discover to what extent FIEBRE sites are affected by arboviral diseases all FIEBRE participant, patients and control serum samples, are tested for: dengue virus, chikungunya virus, Zika virus, Japanese encephalitis virus (for Laos) and o’nyong nyong virus (African sites). The confirmatory arbovirus testing takes place at the French National Centre of Reference of Arboviruses, Unité des virus Emergents (UVE) affiliated with Aix-Marseille University, Institut National de la Santé Et de la Recherche Médicale (INSERM) and Institut de Recherche pour le Développement (IRD).
UVE tests the acute (day 0) and convalescent (day 28) sera. This comprises one sample at day 0 for controls and two samples to test at D0 and D28 for patients.
Processes at UVE:
- samples scanned and recorded onto database to sort the samples per participant, pairing the D0-D28 samples and recording patient type/date sample taken
- tubes decapped and transferred to 96-well microplate ready for extraction or ELISAs
- serum tested for viral RNA using real-time reverse transcription polymerase chain reaction (RT-PCR)
- specific ELISAs used to look for serological evidence of infection
- fully automated ELISA test kits processed - 15 plates in a single run
- further testing with microneutralisation is conducted to distinguish between any co-detection or cross reaction
- once results validated automatically exported to database.
The majority of respiratory tract infections are caused by a variety of commonly occurring viruses. As patients often present with a number of overlapping signs and symptoms it can be difficult to determine the source of the infection by clinical presentation alone. Therefore in order to detect the prevalence of respiratory infections within the study population, pharyngeal swabs are collected from all participants and controls to be screened diagnostically.
At each site participant swabs are frozen and stored in -80°C freezer. The specimens are shipped to LSHTM then on to Micropathology Ltd.
Micropathology Ltd performs diagnostic respiratory screening on the participant swabs to determine which respiratory viruses are present. The Luminex panel screens for: influenza A and B and respiratory syncytial virus (the multiplex assay also detects human metapneumovirus, parainfluenza virus types 1, 2, 3 and 4, adenoviruses, rhinoviruses, enterovirus, parechovirus, bocavirus, Mycoplasma pneumoniae, and other potential respiratory pathogens).
Screening procedures:
- Throat swabs from study participants are received at Micropathology Ltd on dry ice. The standard specimen is a dry, flocked cotton throat swab head in a 2 ml tube. These are inventoried and transferred to -80◦C storage.
- Specimens are thawed and racked according to LSHTM electronic lists. Working in a BSL II biosafety cabinet, 500 uL of buffer is added to resuspend the dry throat swabs. The tubes are recapped and vortexed thoroughly to ensure any pathogen material is resuspended in the swab media.
- An extraction protocol is performed to purify and recover DNA and RNA from 200 uL of swab media. Micropathology Ltd scientists have worked with experts at Promega to optimise a highly effective extraction chemistry. The custom Promega chemistry is run in 96-well format using an open KingFisher™ Flex robotic platform (Thermo Scientific™).
- Diagnostic respiratory screening is performed using the NxTAG® Respiratory Pathogen Panel (IVD) from Luminex (RPP). The RPP kit allows for the simultaneous detection of 18 viral and 3 bacterial gene targets. The bench process involves an initial PCR and bead hybridisation phase on a thermal cycler. Analysis of bead-associated pathogen amplicon is performed using a Luminex MagPix® instrument. A comprehensive report is prepared.
- Output files are exported and reviewed by Micropathology Ltd scientists. Any specimens positive for the NxTAG® combined Rhinovirus/Enterovirus output undergo a further nested PCR test for species differentiation.
- All specimens taken from 1 January 2020 undergo a separate in-house PCR test for SARS-CoV-2 (UKAS accredited test).
- Data from each site is collated and quality controlled at Micropathology Ltd. Once screening for all participants at a site is complete, the results are made available to the consortium.

Laboratory manager at Micropathology Ltd preparing pharyngeal swab samples for respiratory testing in a BSL II hood
Mycobacterial bloodstream infections are a rare, but serious, cause of fever. HIV-positive people are more at risk of mycobacterial infections. A mycobacterial blood culture is performed for inpatients aged >15 at study sites where HIV prevalence in the general adult population is known to be >1% (Malawi [outpatients also included], Mozambique and Zimbabwe).
Site laboratory staff identify the mycobacteria positive cultures; all results are reported to the patient’s clinical team. For external quality assurance, a sample of each isolate is then shipped directly to the National Reference Center (NRC) for Mycobacteria, Germany, a Supranational Reference Laboratory (SRL) of WHO. The NRC participates in the coordination of measures in the fight against and the surveillance of tuberculosis (TB). It examines approximately 12,000 samples a year for the detection and identification of mycobacteria as well as for susceptibility testing.
Process on site (see SOPs):
- where sites do not have access to a Bactec machine, Bactec bottles incubated manually (bottles contain a sensor at the bottom that changes fluorescence with increasing CO2 concentration):
- bottles examined on day 3, 4 or 5 then weekly for six weeks
- compare fluorescence with a positive control bottle
- return negative bottles (no fluorescence) for further incubation
- if positive, small volume of culture supernatant removed to stain
- mycobacteria observed, grown and identified
- positive mycobacterial blood culture cryopreserved in duplicate, one for NRC one for study site
Quality assurance testing procedure at NRC involves:
- differentiation of mycobacteria by means of different molecular methods based on nucleic acid amplification techniques in a stepwise manner
- susceptibility testing of all mycobacteria species in liquid media
- detection of mutations associated with resistance against first- and secondline drugs
More details about the scope of NRC’s work is available on their website.

Even though rapid diagnostic testing (RDT) for malaria has revealed that most febrile patients in Africa and Asia do not have malaria, FIEBRE still needs to confirm this by testing all participants at the point of care. In order to standardise diagnostic testing further external quality confirmatory (EQC) assessment of site results are performed at the Clinical Diagnostic Parasitology Laboratory (CDPL) at Liverpool School of Tropical Medicine.
Many stages are involved in testing for malaria starting from the point when a patient is enrolled on site to the final results being logged on the database at LSHTM:
- mRDT performed at patient enrolment
- Positive results reported to clinical team
- Thick and thin blood smear prepared on microscopic slides
- Slides double-read for malaria only (presence or absence, density and species) by in-country microscopists who have achieved expert status in the WHO (or similar) qualification scheme.
- In-country microscopists read the smears for all mRDT-positive and 10% of mRDT-negative study participants. FIEBRE uses a combination mRDT that detects two different parasite antigens: histidine-rich protein 2 (HRP2) which is produced only by Plasmodium falciparum parasites; and pan-specific Plasmodium lactate dehydrogenase (pLDH) which is produced by all malaria parasite species.
- Site coordinators select 10% of all slides collected at their site for EQC at the CDPL, to be read for malaria and also for Borrelia spp and other pathogens
- Slides are received in by the LSHTM team who distribute to the reference laboratories
- CDPL receive stained thick and thin blood smears where they are checked and catalogued
- Each slide is read by two Health and Care Professions Council (HCPC) registered biomedical scientists with experience in reading blood smears. They are examined for the presence or absence of malaria, borrelia and any other relevant blood parasites. The smears are read blind without the knowledge of the results from the originating site.
- Results are recorded for each slide and once complete the data is submitted to the FIEBRE co-ordinator at LSHTM.

Site activities essentially finished during 2021 as recruitment ended and sample shipments were despatched. The main work now revolves around team collaboration on data queries and analysis of findings.
The final participant samples (sera, blood, plasma, blood cell pellet, buffy coat, PAXgene tubes, swabs) arrived at LSHTM from Laos and Mozambique in November 2021. Microbiology and mycobacteriology isolates were shipped directly to international reference laboratories in Germany and New Zealand.
FIEBRE enrolled child and adult inpatients and outpatients who presented with fever over ≥24 months. A venous blood sample and pharyngeal swabs were collected from all participants; a urine sample was collected from selected participants. In addition, blood and pharyngeal samples were taken from community controls matched to outpatients by age, gender, residence and month of recruitment. The controls were also surveyed to obtain representative data about treatment seeking and medicine use.
Number of patients enrolled and participant characteristics
| Laos | Malawi | Mozambique | Zimbabwe | All sites | |
|---|---|---|---|---|---|
| Total patients enrolled | 1,972 | 1,766 | 2,143 | 1,924 | 7,805 |
| Female | 50% | 56% | 55% | 49% | 53% |
| Male | 50% | 44% | 45% | 51% | 47% |
| Aged <15 years | 39% | 54% | 53% | 46% | 48% |
| Aged ≥15 years | 61% | 46% | 47% | 55% | 52% |
| Outpatient | 48% | 68% | 54% | 58% | 57% |
| Inpatient | 52% | 32% | 46% | 42% | 43% |
| Total controls | 485 | 908 | 559 | 433 | 2385 |
FIEBRE researchers presented their work during ASTMH 2021. Colleagues from all our sites and many partner organisations contributed to the amazing team work enabling us to showcase FIEBRE's achievements so far.
Read the abstract booklet (pdf) and view the posters (pdf).
Talks:
- Development and performance evaluation of a low-cost, high throughput, multiplex immunoassay of 13 fever severity and aetiology markers (pdf)
- Predicting mortality in febrile adult patients: a prospective validation of the MEWS, qSOFA and UVA scores in four health care settings in Africa and South-East Asia (pdf)
Posters:
- Fighting adult mortality through etiology of fever studies: Description of high mortality in an adult inpatient population in Mozambique (pdf)
- The main pathogens causing febrile illness and implications for fever management in Laos; results from the FIEBRE study (pdf)
- Understanding antimicrobial resistance through the lens of antibiotic vulnerabilities in primary health care in rural Malawi (pdf)
- Sex Work, Antibiotic Use and the Management of Sexually Transmitted Infections in Harare, Zimbabwe: An Ethnographic Study (pdf)
- High mortality in adult patients with HIV admitted with fever to hospitals in Malawi, Mozambique and Zimbabwe - results from the FIEBRE study (pdf)
- Negotiating Myanmar’s Law and (Dis)Order amidst Antimicrobial Resistance Policy Implementation (pdf)

The American Society of Tropical Medicine and Hygiene 2021 Annual Meeting (ASTMH 2021) takes place 17-21 November 2021 virtually.
There are eight FIEBRE-related activities covering all aspects of the study (two talks and six posters [pdf]):
Session 84, Clinical Tropical Medicine: Diagnostics and COVID Sat 20 Nov 8:00 – 9:45 am US EST
- Development and performance evaluation of a low-cost, high throughput, multiplex immunoassay of 13 fever severity and aetiology markers, Tegwen Marlais
- Sat 20 Nov, 8:10 US EST
- Predicting mortality in febrile adult patients: a prospective validation of the MEWS, qSOFA and UVA scores in four health care settings in Africa and South-East Asia, Manophab Luangraj
- Sat 20 Nov, 8:20 US EST
Poster Session A Thurs 18 Nov, 11:00 am – 12:30 pm US EST
- Negotiating Myanmar’s Law and (Dis)Order amidst Antimicrobial Resistance Policy Implementation, Yuzana Khine Zaw
Poster Session B, Fri 19 Nov, 12:00 – 1:30 pm US EST
- Fighting adult mortality through etiology of fever studies: Description of high mortality in an adult inpatient population in Mozambique, Justina Bramugy
- The main pathogens causing febrile illness and implications for fever management in Laos; results from the FIEBRE study, Manophab Luangraj
Poster Session C, Sat 20 Nov, 11:00 am – 12:30 pm US EST
- Sex Work, Antibiotic Use and the Management of Sexually Transmitted Infections in Harare, Zimbabwe: An Ethnographic Study, Salome Manyau
Join us online to find out more and share your news from the conference. Tweet @FeverStudies using #TropMed21

Two abstracts from the Zimbabwe team have been accepted as talks for the African Society for Laboratory Medicine 2021 Conference (ASLM 2021).
Mutsawashe Chisenga, FIEBRE medical laboratory scientist, will be presenting on behalf of the team on:
- Detection of antibiotic activity in urine using a bioassay in samples patients with suspected urinary tract infections presenting to primary care clinics
Mutsawashe R Chisenga, Forget Makoga, Beauty Makamure, Heidi Hopkins, Ben Amos, Katharina Kranzer, Ioana D Olaru
-
The use of InTray COLOREX Screen and ESBL for bacterial identification and ESBL-detection from blood and urine cultures in Harare, Zimbabwe
Mutsawashe R Chisenga, Forget Makoga, Gwendoline Chimhini, Beauty Makamure, Heidi Hopkins, Katharina Kranzer, Ioana D Olaru
The conference is taking place virtually from 15-18 November 2021. Programme details to follow.
The MLW team held two successful local dissemination meetings in a COVID-19-safe format for St Montfort Hospital and Chikwawa District Hospital in mid-May 2021. The meetings were attended by representatives from the hospital management teams, lead clinical officers, district health officers, the matrons, and other members of staff from all sections of the hospitals including clinicians, nurses, anaesthetists and administrators.
The meetings covered presentations on:
- Study operations review
- Community field work
- Scientific and statistical data
- Social science key findings
The meetings were very well received and emphasised some basic practice points. Further meetings at a national level will be organised when the reference laboratory results are available later in the year.
The social science team discussed their research from clinical observations and the focus on cotrimoxazole being the most frequently used drug at the facilities and within the community.
The key take home messages were:
• A limited number of antibiotics are recognised and there are very low stocks of antibiotics in public health clinics – not suggesting that antibiotics are overused everywhere
• Health workers are very committed and manage in challenging conditions where essential medicines beyond those provided by the international donor programme are very limited
• Opportunities exist for rapid gains through antimicrobial resistance policy that reflects the context of this limited access and centres on foregrounding care not restricting access to antibiotics.

FIEBRE finished recruiting patients in Malawi on 31 March 2021. Follow up activities will continue during April.
Congratulations to everyone involved in contributing to the study in both Blanytre and Chikwawa. Many thanks to the clinical, laboratory and technical staff, community engagement team, administrators, data scientists, patients and the local community.
The first patient was enrolled on 4 July 2018 at Chikwawa District Hospital (CDH). In total, 1765 patients were recruited (over 6200 screened) and the team reached the outpatient recruitment target of 600 for both adults and children. Control recruitment was very effective at 98% and 28-day follow up was high at 91%. This reflects the dedication of the community engagement team.
Initially, FIEBRE started at CDH and second site opened at St Montfort Mission hospital in May 2019 in order to help increase inpatient recruitment.
Community engagement was integral to the study. FIEBRE was helped by long-established community sensitisation work to research in the area. There's been particular interest and concern about the amount of blood taken. These issues are addressed by the team through their continued commitment to working with the local community.
The team faced many challenges such as flooding, Cyclone Idai in March 2020, the COVID-19 pandemic and a high number of eligible patients declining to participate for a variety of reasons including:
- Concern about donating blood/large volume when anaemic (severe anaemia is commonplace in Chikwawa)
- Traditional beliefs about losing and sharing blood
- Wanting to seek consent from husband or elder
These sensitive issues were addressed over the course of the study by many staff and patient education and sensitisation sessions explaining the safety and desirability of blood testing in patients who might be suffering from infections. On the whole, these efforts were bearing fruit with increased inpatient recruitment until the arrival of COVID-19 in the spring of 2020.
The COVID-19 pandemic had a considerable impact on the study’s activities; national and institutional restrictions were imposed which slowed down enrolment and limited activities. Although personal protective equipment was available for staff, and infection control measures were in place, with masks and screening at the hospital entrance, people were reluctant to seek healthcare due to a fear of COVID-19 transmission in health centres and of being diagnosed and being sent to a COVID-19 treatment centre.
Despite all the challenges, the FIEBRE team have been able to help support and add to the overall level of care and treatment in the area by providing clinical microbiology services in CDH and SMH for the first time and diagnosing HIV and TB in patients who might otherwise have slipped through the net. FIEBRE staff followed up any positive blood cultures immediately and advised clinicians on antibiotic choice and length of treatment. The study hopes to leave a legacy whereby this service will be continued by the MLW core team.
Many of the FIEBRE team members in Malawi were undertaking their first research role. It has been rewarding to see the team develop and no surprise to see team members grow into more senior roles or be taken up by other studies, research support units or research institutions in Malawi.

Images of the MLW team working in the hospital and field (2018-21)
FIEBRE finished recruiting patients in Mozambique on 26 February 2021. Control and follow up activities will continue throughout March.
CISM began enrolling adult patients at Manhiça Health Research Centre on 13 March 2019 after paediatric recruitment started in November 2018. A second site, Hospital Geral José Macamo in Maputo, was opened on 28 November 2019. This helped boost inpatient numbers significantly and improved the patients’ care with access to blood culture results.
In total, 2136 patients were enrolled during this period; the highest number of participants of all the sites. This is a great achievement. The team reached the outpatient child recruitment target of 600. The site is the only one which has recruited participants who tested positive for SARS-CoV-2.
Congratulations to everyone involved in contributing to the study’s success - the clinical, laboratory and technical staff, administrators, data scientists, patients and local communities.
Initially, there were some clinical issues which included difficulty in taking blood and urine samples from children. Day 28 follow up has been quite problematic with a high rate of refusals and some loss to follow up due to migration (families moving for work), accessibility during the rainy season and local perception of loss of blood. Blood extraction in particular is a sensitive issue and can be associated with ill-health especially among children. Moreover, some healthy people were not interested in participating as controls.
The COVID-19 pandemic had a considerable impact on the study’s activities; inpatient recruitment was closed down in Maputo and community activities were stopped during the first wave. National and institutional restrictions were imposed which slowed down enrolment and limited field activities.
On a personal level, many of the clinical staff were infected with SARS-CoV-2 although none severely. This was a critical period during which infection protection measures and disinfection of working areas were reinforced. It was a stressful time for all the staff and their families. Despite these COVID-19 issues along with rising patient numbers and infections in the community, the team managed to continue working throughout.
In spite of the many challenges, the team’s hard work and dedication has contributed greatly to the clinical diagnostic capacity at these hospitals through access to rapid diagnostic tests and laboratory cultures. This has benefitted both clinicians and patients, enabling clinicians to use the information to make accurate diagnoses and therefore quickly help provide the right treatment.
Listen to Justina Bramugy and Campos Mucasse talking about their experience on FIEBRE.

The LOMWRU team returned to the study site at Vientiane Provincial Hospital on 19 January 2021 for the main dissemination meeting. The preliminary results of the study, some microbiology data and early reference laboratory results were presented to clinicians, nurses and laboratory technicians for feedback.
The 2-hour session was chaired by Dr Siho Sengsavang, Deputy Director of Vientiane Provincial Hospital and Prof Mayfong Mayxay, Vice President of the University of Health Sciences, LOMWRU Head of Field Research and local PI who facilitated a discussion after talks by Dr Manophab Luangraj and Dr Vilayouth Phimolsarnnousith.
Watch a video summarising the study's activities.

The BRTI team held a series of successful dissemination meetings following the completion of recruitment in Zimbabwe on 30 September 2020,
Preliminary clinical results were presented at the polyclinics during October and November for the nurses and staff involved in FIEBRE. These received interesting feedback and led to discussions about effective antimicrobials and antimicrobial resistance.
A research dissemination meeting for major stakeholders at a policy level took place in Harare (with online attendees) on 30 November. The meeting covered several projects as well as FIEBRE and ARGUS: B-GAP, FAST and BREATHE trial.
Representatives from the Ministry of Health and Childcare, Harare Central Hospital, Parirenyatwa Hospital, Medicines control authority of Zimbabwe, Zimbabwe College of Public Health Physicians, National Microbiology Reference Laboratory, MSF, UNAIDS and many other institutions participated in the meeting.
The FIEBRE and ARGUS presentations are available:
- Overview of FIEBRE: Evaluating causes of fever and antimicrobial resistance in sub-Saharan Africa and Southeast Asia (pdf), Heidi Hopkins
- FIEBRE: Preliminary results Ioana Ularu, Molly Sibanda
- The Zimbabwe typhoid outbreak & impact of vaccination on typhoid cases and antimicrobial resistance (pdf), Mutsawashe Chisenga
- FIEBRE Understanding the social context of antibiotic prescribing practices and antibiotic use in Zimbabwe (pdf), Salome Manyau, Justin Dixon
-
ARGUS: Prevalence of Gram-negative antimicrobial resistance in patients presenting with urinary tract infections in primary care facilities in Harare (pdf), Ioana Olaru
Recruitment of FIEBRE participants ended in Laos on 31 October 2020. The LOMWRU team started enrolling patients on 9 October 2018 at Vientiane Provincial Hospital. In total, 1961 participants were enrolled during this period. The team reached the adult recruitment target of 600 for both in- and outpatients.
Congratulations to everyone involved in contributing to the study’s success - the clinical and laboratory staff, hospital, participants and local communities.
The first set of samples were shipped to LSHTM in early 2020 and are now at international reference laboratories awaiting diagnostics. Analysis of these samples will produce the first results of the study aside from preliminary data from point-of-care tests carried out on site. The dried blood spots from Laos are also currently being used for the MOS-DEF biomarker project.
The team faced clinical and logistical challenges including difficulty in taking blood samples from children and travelling long distances to recruit controls (healthy people who were not always interested in taking part). The team continued working throughout the COVID-19 epidemic despite national restrictions which slowed down enrolment and limited field activities.
FIEBRE has helped with the clinical diagnostic capacity and treatment of infectious diseases in the local community, as blood culture and other tests were not available in the hospital previously. The information collected by the study may contribute to the development of treatment guidelines for fever and antibiotic stewardship in the future, especially in settings where there's limited laboratory diagnostics or little data available.

The American Society of Tropical Medicine and Hygiene 2020 Annual Meeting (ASTMH 2020) took place 15-19 November 2020 virtually.
There are four FIEBRE-related activities (two presentations and two posters):
-
Change in Salmonella Typhi incidence and antimicrobial resistance patterns following mass vaccination with the new typhoid conjugate vaccine (pdf), Ioana Olaru
-
Scientific Session 35: Bacteriology: Systemic Infections Session
-
Tuesday 17 Nov 9.00 am - 10.45 am US Eastern Time Zone
-
-
Antibiotic use among residents in Uganda, Zimbabwe and Malawi – a mixed methods study (pdf), Clare Chandler
-
Scientific Session 152: Global Health: Maternal, Newborn, Child Health and Neglected Tropical Diseases
-
Thursday 19 Nov 3.00 pm - 4.45 pm US Eastern Time Zone
-
- Evaluation of the CompactDry EC culture plates for the diagnosis of urinary tract infections in Harare, Zimbabwe (pdf), Ioana Olaru
- Poster Session A, Monday, 16 November
1.30 pm – 3 pm US Eastern Time Zone
- Poster Session A, Monday, 16 November
- Preliminary findings from the FIEBRE study in Mozambique: clinical findings and results of point-of-care tests and microbiology studies (pdf), Marta Valente
- Poster Session B, Tuesday, 17 November
11.45 am – 1.15 pm US Eastern Time Zone
- Poster Session B, Tuesday, 17 November
Join us online to find out more and share your news from the conference. Tweet @FeverStudies using #TropMed20

Participant recruitment in Zimbabwe finished on 30 September 2020. Many congratulations to everyone involved in contributing to the success of the study - the collaborators, participants and local communities.
Zimbabwe was the first FIEBRE site to start operating in June 2018. In total, 1923 participants were enrolled during this period. FIEBRE recruited patients presenting with fever to three major hospitals and three polyclinics in Harare.
The team at Biomedical Training and Research Institute (BRTI) continued working through outbreaks of cholera and typhoid, political and economic difficulties, despite all these challenges the outpatient adult target (of 600) was reached and many patients received life-saving diagnostics and treatment that otherwise they may not have been able to access. Through the study, a large number of patients with typhoid fever were diagnosed and received effective treatment. In addition, patients with other life-threatening infections such as tuberculosis, malaria and cryptococcal meningitis could be diagnosed.

Photos of team members working in Harare (before and during COVID-19)
A series of papers by Infectious Diseases Data Observatory (IDDO) that set out to explore the global distribution of infections that cause non-malarial febrile illness (NMFI) has been published in BMC Medicine.
The series was a collaboration by scientists and researchers from institutions across the world (including FIEBRE Pis from LSHTM, LOMWRU and University of Otago) who conducted large-scale systematic reviews of published literature to map the cause of febrile illness, once malaria had been excluded. Historically, malaria was assumed to be the cause of fever, however, the advent of rapid diagnostic tests, combined with intensified malaria control activities over the last decade, has substantially reduced incidence rates and it is now clear that most acute fever cases are of non-malaria aetiology.
The results of these systematic reviews, covering Africa, Latin America, and Southern and South-Eastern Asia, have been incorporated into an open-access online database that supports an interactive map that can filter data by country, microorganism type, patient age, sample type, pathogen family, genus and species, study year, geographic region and sub-region.
The collection of articles are available on BMC Medicine. Read more details on the IDDO website.
Non-malarial febrile illness: a systematic review of published aetiological studies and case reports from Africa, 1980–2015
Jeanne Elven, Prabin Dahal, Elizabeth A. Ashley, Nigel V. Thomas, Poojan Shrestha, Kasia Stepniewska, John A. Crump, Paul N. Newton, David Bell, Hugh Reyburn, Heidi Hopkins and Philippe J. Guérin
Febrile illness mapping—much of the world without data and without evidence-based treatments
Paul N. Newton and Philippe J. Guerin
When fever is not malaria in Latin America: a systematic review
José Moreira, Janaina Barros, Oscar Lapouble, Marcus V. G. Lacerda, Ingrid Felger, Patricia Brasil, Sabine Dittrich and Andre M. Siqueira
Non-malarial febrile illness: a systematic review of published aetiological studies and case reports from Southern Asia and South-eastern Asia, 1980–2015
Poojan Shrestha, Prabin Dahal, Chinwe Ogbonnaa-Njoku, Debashish Das, Kasia Stepniewska, Nigel V. Thomas, Heidi Hopkins, John A. Crump, David Bell, Paul N. Newton, Elizabeth A. Ashley and Philippe J. Guérin
Researchers from the Anthropology of Antimicrobial Resistance (AMIS) team at the LSHTM work in countries across Africa and Asia, including Ghana, Uganda, Malawi, Zimbabwe, China, Myanmar and Thailand, to understand how antibiotics are used in society. The FIEBRE social science team has contributed valuable insights into this work on antibiotics and the rise globally in antimicrobial resistance. See what their research has discovered about the differing roles of antibiotics in Malawi and Zimbabwe.
The FIEBRE protocol paper is now available on BMJ Open:
Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE): protocol for a multisite prospective observational study of the causes of fever in Africa and Asia
Heidi Hopkins, Quique Bassat, Clare IR Chandler, John A Crump, Nicholas A Feasey, Rashida A Ferrand, Katharina Kranzer, David G Lalloo, Mayfong Mayxay, Paul N Newton, David Mabey, and FIEBRE Consortium co-authors
This paper provides an overview of FIEBRE’s clinical, epidemiological and laboratory activities. The study aims to identify infections that are treatable and/or preventable, to assess antimicrobial susceptibility of bacterial pathogens, to evaluate host response biomarkers that may help in predicting illness severity and distinguishing bacterial from other causes of fever, and to collect qualitative data on care-seeking and treatment behaviours in each study area.
The paper details the harmonised study design, clinical and laboratory assessments, and data analysis plan; gives information about the sites; and outlines the study’s strengths and limitations. Listen to Prof David Mabey introduce the paper:
It is with great sadness that we announce the sudden death last week of our dear friend and former colleague Dr Amit Bhasin, who was our FIEBRE programme manager from 2016-2019. Amit first joined the School in 2002 as manager of the Gates Malaria Programme, and moved to Cambridge University in 2019 to run the Cambridge-Africa Programme. He will be greatly missed by his many friends around the world. Read the full tribute to Amit here, find out how to send your condolences to his family, about a memorial scholarship and other memorial events to be arranged in the future.

Read about Ja Seng Bawk's experience in quarantine when she returned home to Myanmar in March. She works as a Research consultant for FIEBRE social science in Myanmar.

On 22 March 2020, I cut my trip to London for the FIEBRE Social Science Analysis meeting short due to the COVID-19 pandemic and ensuing lockdowns. When I arrived at Yangon International Airport, no one asked for a medical certificate (although this was stated as a requirement on the official website, resulting in me spending the last two days in London in a futile effort to acquire one). Instead, I had to fill out a Health Declaration card, which was handed out on the plane and I gave to the airport authorities on arrival. They gave me the choice of a public medical centre free of charge or a hotel at 7 MMK lakhs (around 500 USD) up front in cash for the 14 days of quarantine. I chose the hotel, which was assigned by the authorities at the airport.
During my time in quarantine at the hotel I was able to join the FIEBRE meeting online and work on transcription. I felt very safe as I was tested for fever and symptoms every morning by a team of about 2 doctors and 3 nurses wearing full personal protective equipment. Food was delivered to the hotel door. I also received approximately four phone calls a day from a department of the Ministry of Health and Sports asking me about my daily condition during quarantine. Because of the very limited availability of PCR testing at the time in the country, I wasn’t tested. After 14 days with no symptoms, I finally returned to my home, safe and sound.
Shortly after this, Yangon went into lockdown. I had to stop all fieldwork and since then, I have been focusing on transcription of interviews at home. As of today (29 May 2020), Myanmar authorities have reported 206 confirmed COVID-19 cases and six deaths since the first cases were reported on 23 March 2020.

The team at Centro de Investigação em Saúde de Manhiça (CISM) in Mozambique have been operational for a year. CISM began enrolling adult patients at Manhiça Health Research Centre on 13 March 2019 after paediatric recruitment started in 23 November 2018. They reached their 1000th patient in November 2019.
Recruitment started at a new site, Hospital Geral José Macamo, Maputo, on 28 November 2019. This has helped boost inpatient numbers considerably and improved the patients’ care with the performance of blood culture, urine culture and antibiotic susceptibility testing. Latest data from end of February shows 1406 patients enrolled of which 916 are outpatients and 490 inpatients. More children than adults have been enrolled.
The team has faced several recruitment challenges during the study. Over the years, inpatient numbers have dropped at Manhiça District Hospital and there has been overlap with other studies making inpatient recruitment particularly difficult.
Day 28 follow up has been challenging with a high rate of refusals and some loss to follow up due to migration, accessibility and local perception of loss of blood.
Throughout the study, the team has received positive feedback from both patients and the hospital. FIEBRE has contributed to the clinical diagnostic capacity at these hospitals through access to rapid diagnostic tests and laboratory cultures. This has helped both clinicians and patients, enabling clinicians to use the information to make an accurate diagnosis and therefore provide the right treatment.

The third FIEBRE Co-investigators meeting, hosted by LOMWRU, took place in Vientiane, Laos from 11-14 February 2020.
Over 45 members of the partner organisations involved in FIEBRE attended the meeting including London School of Hygiene & Tropical Medicine, Barcelona Institute for Global Health (ISGlobal), Biomedical Research and Training Institute (BRTI), Centro de Investigacao em Saude de Manhica, Liverpool School of Tropical Medicine, Malawi-Liverpool Wellcome Trust Research Programme, Mahidol Oxford Tropical Medicine Research Unit (MORU), University of Oxford, Bangladesh Institute of Tropical & Infectious Diseases and Chattogram Medical College Hospital.
Researchers from the Aga Khan University also joined the meeting to learn more about the possibility of setting up a similar study in Karachi, Pakistan.
The event was attended by representatives from all aspects of the study - data, clinical, laboratory, microbiology and social science. There was a packed agenda covering updates on:
- Country sites - personnel, number of patients and controls recruited, logistical recruitment challenges
- Sharing solutions to operational challenges
- Laboratory activities
- Reference laboratories – status on sample shipment
- Microbiology data and early analysis
- Quantitative data analysis plan
- Social science activities
- Complementary and associated studies
- Dissemination of results and engagement
- Publications, follow-on studies and future plans
- Potential new sites
The visit included a tour of the laboratory at Mahosot Hospital and the recruitment site Vientiane Provincial Hospital illustrating how the study operates from the initial recruitment of patients through data input to the samples being tested at the laboratory.
The meeting was very productive enabling all participants to share ideas and benefit from learning about the experiences of partners across sites and disciplines.
Members of the team and the DfID technical representative were also able to meet with the British Ambassador to explain the study.

A series of videos is being recorded of team members. These will give an insight into various different aspects of the study, from the hub at the London School of Hygiene & Tropical Medicine where FIEBRE is co-ordinated to the teams in Laos, Malawi, Mozambique and Zimbabwe who are enrolling the patients, taking samples and collecting the data.
The first video is with Dr Katharina Kranzer, the Principal Investigator in Zimbabwe, who explains how FIEBRE operates with BRTI in Harare.
The LOMWRU team started enrolling patients on 9 October 2018 at Vientiane Provincial Hospital “Maria Teresa hospital” 60 km from Vientiane. The team recruited its 1000th patient in early September.
Latest data from early October show 1165 patients enrolled of which 669 are inpatients and 495 outpatients.
The team comprises three doctors, two who are responsible for recruiting in- and outpatients, and one for control recruitment. There’s one laboratory technician and one person concentrating on 28-day follow-up.
The team has faced many challenges since the start due in part to the weather. Patients present with fever relating to the season. Recruitment increased during the rainy season (May to October) despite only having three doctors during a dengue fever outbreak. The enrolment rate has sped up since recruitment started 24 hours a day/7 days a week with the help of a local assistant doctor. Another challenge for the team is handling children while taking blood from them. Parents are often not willing to give consent.
Day 28 follow up is quite successful at about 80% and varies depending on the weather. Reasons for refusal include feeling much better so there's no desire for further blood tests; moving (work or life -related); geography and distance; and inability to leave work.
Community control recruitment can be like an adventure, as the countryside is very diverse and the distances so far. Some trips can take up to two hours each way. None of the trips are easy and can even involve transporting the vehicle and equipment by boat across a river.
Throughout the study, the team has always received positive feedback from both patients and the hospital. FIEBRE has been able to contribute to the clinical diagnostic capacity of infectious diseases locally, as blood culture and other tests are not available in the hospital.

FIEBRE will attending the 11th European Congress on Tropical Medicine and International Health (ECTMIH) from 16-20 September 2019 in Liverpool, UK.
Members of the team will be presenting on two topics:
- FIEBRE (Febrile Illness Evaluation in a Broad Range of Endemicities): a multi-site prospective observational study of causes of fever in sub-Saharan Africa and Southeast Asia
Hopkins H, Bassat Q, Chandler CIR, Crump JA, Feasey NA, Ferrand RA, Lalloo DG, Mayxay M, Newton PN, Mabey DCW
(Tuesday 17 September, Rm 1a, 14.00 h) - Emergence of diminished ciprofloxacin-susceptibility Salmonella typhi in an ongoing outbreak from Harare, Zimbabwe
Ioana D Olaru, Nicholas Feasey, Rashida Ferrand, David Mabey, Heidi Hopkins, Sekesai Zinyowera, Ben Amos, Katharina Kranzer
(Thursday 19 September, Rm 1a, 15.00 h)

In Malawi, the first patient was enrolled on 4 July 2018; their 1000th patient, a child outpatient, was signed up at Chikwawa District Hospital on 11 June 2019.
The Malawi-Liverpool Wellcome Trust Research Programme (MLW) team initially comprised 11 front line staff, working at Chikwawa District Hospital, rising to 15 in April 2019, as the second site in Ngabu, was opened to speed up inpatient recruitment, and weekend work started. The laboratory and freezers, where samples are further tested and stored, are based at MLW in Blantyre.
Community engagement is integral to the study's success. FIEBRE has been helped by earlier community sensitisation work in the area. Two of the original fieldworkers are very well known and trusted in the community which has aided acceptance of the study. Before and during the study, there are regular engagement activities with the local community, including meetings with the district council where the local chiefs and villagers ask questions about the study. There's been particular interest and concern about the amount of blood taken (shown to a certain extent in some difficulties with getting blood samples from controls). Issues such as this are addressed by the team and their continued dedication in working with the local community.
Chikwawa district and the Chikwawa district hospital are resource poor and constitute one of the most deprived areas and facilities in the country. The hospital is situated by the Shire river, in a largely rural district. In March, the study had to close for a week due to severe floods. The main road was washed away and one of the villages was badly affected with scores of homes washed away. Many of these people are living in temporary shelters and awaiting support for new homes to be built. The situation was further compounded by Cyclone Idai causing flash floods shortly afterwards. St Montfort Hospital the second site is 30 km away from Chikwawa. This hospital is located in the major town in the district which services the main employer: Illovo Sugar.
In these settings, the FIEBRE team have been able to help support and add to the overall level of care and treatment in the area. Moreover, a clinical microbiology and blood culture service has been provided enabling FIEBRE to contribute to the clinical diagnostic capacity at these hospitals. Even patients not on the study can access this service. Six cases of TB have been diagnosed in patients who otherwise would not have been diagnosed. This is likely to have been life-saving for some. There have been 16 positive blood cultures indicating septicaemia or blood poisoning. This is often fatal but thus far the patients have survived demonstrating further benefits to the community.
Data for the year shows almost 4700 patients have been screened with about 1100 recruited. Loss to 28-day follow-up is at 7%, which reflects the good work of the team, as it can be challenging with inpatients as some reside outside the usual catchment area.

A year ago, the BRTI team in Zimbabwe started recruiting patients for FIEBRE. The first participant signed up for the study was a child outpatient enrolled on 22 June 2018. Since then more than 1000 participants have been recruited from five clinics across Harare and Chitungwiza. The team has diagnosed a large number of typhoid fever cases and collected invaluable data about the extent of antimicrobial resistance. Patients have received life-saving treatment that otherwise they may have had difficulties in accessing. FIEBRE has contributed to the clinical diagnostic capacity at these sites and also to regional antimicrobial resistance surveillance efforts.
Our dedicated field staff, comprising eight nurses and six research assistants, are currently working in two major hospitals (Harare Central and Chitungwiza General), three primary care clinics (Budiriro, Glen View and Rutsanana Polyclinics) and their surrounding communities. Three MSc students (two from LSHTM and one from University of Oxford) are helping with various aspects of the study. Felicity Aiano is working with the social science team; Michael Blank is working in the laboratory on the ARGUS study; and Zay Yar Aung has been investigating antimicrobial prescribing at Budiriro clinic.
The latest data show out of almost 1800 patients screened, 1038 were enrolled. The reasons for ineligibility vary and are monitored to establish if there are steps that can be taken to aid recruitment such as encouraging patients to consent through raising local community awareness of the study and its potential benefits. The amendment to the protocol to discard 'elevated respiratory rate' and 'cough' as an exclusion criterion for outpatients <15 years has helped recruitment in this category. Outpatient recruitment has gradually increased, particularly with the opening up of new sites in April. Control recruitment has been steady (67% of selected outpatients have been matched and recruited). The loss to follow up for Day 28 recruitment is low with 92% of patients having a Day 28 visit.

Recruiting a community control is not not as simple as it seems. There's many stages involved behind the scenes as community controls must be frequency matched to the cases by age, sex, seasonality and geographical area. These data are calculated by the team at LSHTM. The LOMWRU team in Laos then spends a considerable amount of time and effort travelling long distances to locate and ask healthy individuals to take part in the study as community controls.
Dr Somvai Singha produced a video of one such trip, which took 2 hours by motorbike and boat, to a village in Viengchan province 90 km away from the capital Vientiane. This shows the lengths people will go to seek care, the same journey can take 4 hours by road.
Watch this fascinating video of a journey to locate a control:
Read about a study participant's experience in Malawi.
Fletcher Nangupeta and Frank Mlumbe, FIEBRE fieldworkers, organised a visit to meet two FIEBRE study participants, a mother and daughter, and the village elder, at William village, in Chikwawa district. The mother explained how her daughter had been enrolled in the study when she had presented with a fever, how the daughter had recovered after treatment and subsequently, when the mother herself became ill with a fever, she had no hesitation in taking part.
FIEBRE in practice: Malawi FIEBRE visit to William village, T/A Katunga
Report produced by Fletcher Nangupeta and Frank Mbalume
On 8 February 2019, we received three visitors from London School of Hygiene & Tropical Medicine namely; Amit Bhasin, Ruth Lorimer and Rebecca Handley. Staff from the Malawi Liverpool Wellcome Trust Research Programme (MLW) were also in attendance: Edward Green, Kate Haigh, Fletcher Nangupeta and Frank Mlumbe
We visited William village, in the traditional authority (T/A) area of Katunga, where we went to the house of a FIEBRE study participant. The main purpose of the visit was to find out what she understood about the study and to see how people in the community perceived the FIEBRE study. On arrival at the village, we were received by the village headwoman herself, the participant and her children. The team was introduced to both the participant and the village headwoman.
When she was asked about how she feels taking part in the study, she said ‘’it is a good experience to participate in the study, this is my second time taking part in the study and two of my children also took part in study”. She appreciated that now she knows the status of her body and the general health of the children. When asked about the blood volume and blood stealing myths which can occur in Malawi, she said, ‘’The myths were circulating sometime ago but nowadays people realise the importance of being in the study”. One inquisitive neighbour had enquired about FIEBRE: a lady from a village nearby who came and asked her if any of her children became sick or were weak after participating in the study and having their blood taken. She assured the lady that the child would be fine and she should not get worried.
The team was interested in what people in the village do when they get sick; the participant and the village headwoman explained that a few individuals buy medicine mainly painkillers from within village shops while many of them go to the hospital when they became sick.
The team asked about the demographic and ethnic composition of the village. The village headwoman said that there are about 300 households in the village mainly headed by males apart from few households which are headed by females and children, and majority of them are from the Mang’anja tribe. The majority of the inhabitants of William village rely on farming as their main economic activity almost every household has a piece of land for farming and others keep livestock such as cattle, goats and chicken. Apart from farming, a few individuals have other jobs while others have small scale businesses.
One of the visitors asked how the village had changed over the years. The participant said that in the past most of the houses were grass thatched whereas now people are able to build iron-roofed houses, and people used to drink water from the wells and river and needed to walk long distances to get water, but now water comes from taps and boreholes.
The meeting concluded after approximately one hour with friendly farewells and handshakes.

Meeting with FIEBRE participants and village elder
All four sites, Laos, Malawi, Mozambique and Zimbabwe, are now operating fully recruiting both adult and children out- and inpatients.
The team at Centro de Investigação em Saúde de Manhiça (CISM) in Mozambique began enrolling adult patients at Manhiça Health Research Centre in mid-March after paediatric recruitment started in December 2019.
Two sites have now recruited over 500 patients. In January, Zimbabwe became the second site following Malawi. To enable enrolment of the full cohort expansion is under way in Malawi and Zimbabwe where nurses and research assistants have been taken on to recruit participants at new sites. The plan is to train staff and be ready to open up the new sites in April.
All sites receive regular recruitment reports tailored to their specific data requirements providing details about the participants, number of controls, 28-day follow ups etc. These updates enable the teams to chart their progress and identify any potential issues.

CISM team

The second FIEBRE Annual co-investigators meeting was held in Zimbabwe from 3 - 6 December 2018. World-leading fever experts were involved in the meeting contributing to the discussions and plans for the study going forward.
Over 40 team members of the partner organisations attended the meeting including London School of Hygiene & Tropical Medicine, Liverpool School of Tropical Medicine, Barcelona Institute for Global Health (ISGlobal), Universities of Oxford and Otago, Centro de Investigacao em Saude de Manhica, Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit , Malawi-Liverpool Wellcome Trust Research Programme and the Mahidol Oxford Tropical Medicine Research Unit.
The Biomedical Training and Research Institute (BRTI) hosted the event attended by representatives from all aspects of the study - laboratory, technical, data, clinical, microbiology and social science.
There was a packed agenda covering:
- Country updates - site and personnel details, number of patients and controls recruited, logistical recruitment challenges
- Solutions to ethical and operational issues
- Open Data Kit
- Laboratory activities
- Update on protocol amendments and implications of the changes
- Reference laboratories
- Microbiology data and interim analysis
- Social science update on overall research questions and initial findings from fieldwork
- Complementary studies
- Dissemination of results
- Way forward, follow-on studies and future plans
A visit to the Harare sites, Budiriro polyclinic, Chitungwiza General Hospital and Harare Central Hospital, and to the BRTI facilities, illustrated how the study proceeds from the initial recruitment of patients through data input to the samples being tested at the laboratory.
The social science team held a subsequent meeting from 7-8 December to look at their progress and analyse the data collected so far.
The meetings were successful and resulted in detailed plans for the study's future.
FIEBRE will be at the 67th Annual Meeting of the American Society of Tropical Medicine and Hygiene (ASTMH) which takes place from 28 October to 1 November 2018 in New Orleans.
Several FIEBRE colleagues are participating in a symposium:
100,000 papers later: Reviewing and mapping the etiology of non-malarial febrile illnesses globally
Session 39, 29 October 2018, 16:00-17:45 h
Organizer: Philippe J. Guerin, Infectious Diseases Data Observatory (IDDO)
More details available on the ASTMH website.
A co-investigators meeting will also be held with representatives from many of the partners and organisations involved in the study taking the opportunity to discuss progress so far.
FIEBRE is progressing well with three sites now enrolling participants and one prepared, ready to start.
October is a busy month with the London School of Hygiene & Tropical Medicine team travelling from Asia to Africa to hold training first in Laos then Mozambique.
The LOMWRU team in Laos, led by Dr Vilada Chansamouth, enrolled their first patient at the Vientiane Provincial Hospital on 11 October 2018.
The background to this was several weeks' of training in all aspects of the study at the MORU office in Laos and at CISM Manhica, Mozambique. This includes reviewing SOPs, data input with ODK, sample taking with colleagues, consent procedures and laboratory management. A range of clinical, technical, data and laboratory staff are all involved.
After gaining experience from previous training and pilot sessions in Zimbabwe and Malawi the activities were adapted to meet the needs of the teams, as all the facilities and personnel involved have different specialisms and knowledge.
Issues have arisen as enrolment is ongoing. Analysis has shown that excluding children with symptoms of cough/fast breathing (only outpatients) has significantly limited recruitment in Zimbabwe, with the potential to do so in Laos and Myanmar. Therefore amendments to the selection criteria have been made to address this.
Questions have also arisen over the definition of fever and methods used by staff (axillary or tympanic measurement of temperatures) for study enrolment, as a result, the SOP is being adjusted to aid enrolment.
The quick access to data through OpenDataKit from all sites enables such issues to be picked up swiftly, discussed and addressed to ensure the study continues to run smoothly, efficiently with consistency from country to country.
FIEBRE is holding a symposium during LSHTM week.
Fever, diagnosis and antimicrobials: the multi-disciplinary FIEBRE study
Date: Monday, 17 September 2018 Time: 14.00–16.30 h
Where: Manson Theatre, London School of Hygiene & Tropical Medicine
The symposium will look at the challenges facing a large multi-disciplinary study taking place across sub-Saharan Africa and South East Asia.
The topics under discussion will be anthropology, paediatric and adult clinical care, antimicrobial resistance, standard and novel diagnostics, approaches to database management, project management and statistical issues.
This will be an opportunity to come along and find out about the latest data emerging from FIEBRE on the causes of fever, antimicrobial resistance, treatment seeking, case management and the roles of antibiotics.
Representatives from the Malaria Centre, AMR Centre and MARCH Centre will be presenting on several aspects of the study including: social science; clinical value of biomarkers; use of and experience of ODK to enable real-time data access; and recruitment of controls.
Speakers include: Shunmay Yeung; Heidi Hopkins; Clare Chandler; Sham Lal; John Bradley; Ioana Olaru and David Mabey.
A recording of the event is available online.
The first patient was enrolled in the study on 22 June 2018 by the team at Biomedical Research and Training Institute (BRTI) in Harare.
The plan is to enrol 2400 febrile patients at each of five sites, over a 12-month period at each site to account for possible seasonal variation. The patients will include children and adults, both inpatients and outpatients.
On the day of enrolment a venous blood sample is taken, a nasopharyngeal swab (to test for respiratory viruses) and in addition a urine sample from young children and patients of any age who have possible urinary tract infection symptoms. At the initial assessment stage, the team will, also undertake diagnostic tests (such as malaria tests and blood culture) that are of immediate clinical benefit to the patient, and for research purposes.
The focus of the study is on infections that are treatable and/or preventable. Participants will also be asked to return in 28 days’ time when a further blood sample will be taken for serology. Community control participants are also recruited to provide additional data for interpretation of diagnostic tests, and information on antimicrobial use in the surrounding communities.
Although fever is one of the most common presenting symptoms there have been few studies on its potential causes. FIEBRE will provide valuable data to help identify the causes of fever in settings where diagnosis is not usually available in real time, thus enhancing the quality of local care.
Recruitment of patients at the Malawi site in Chikwawa is due to begin in the coming weeks, following training sessions and collaborative work to refine data collection tools and to address site-specific logistical aspects.
Final FIEBRE study preparations are underway with formal staff training at the Biomedical Research and Training Institute (BRTI) in Harare. Staff from Malawi, Zimbabwe and LSHTM assembled for collaborative training, bringing together all components of the study: clinical, laboratory, social science, and data teams. The teams reviewed the study protocol, standard operating procedures (SOPs), and data forms, and discussed and practiced harmonised procedures for obtaining informed consent, collecting clinical and laboratory data, and obtaining samples from FIEBRE study participants.
The clinical team practiced recording information using the Open Data Kit (ODK) data entry system. The social science team joined clinical colleagues to share advice on administering aspects of study questionnaire.
Following training at BRTI, the LSHTM team moved on to Malawi to continue work and training with collaborators at the Malawi–Wellcome Trust’s (MLW’s) facilities. The collaborative work across sites is important to identify and address any potential issues before the study begins in earnest, and to ensure consistency between sites.
The FIEBRE scientific coordinator, Dr Heidi Hopkins, said, “The site staff have shown great teamwork, expertise, focus, and engagement during this training period in Zimbabwe and Malawi. Together, we’ve been able to identify and address many questions and challenges while beginning to operationalise the protocol on the ground. Pilot enrolment will begin at both sites as soon as a few final details are tucked in, and staff training in Laos, Mozambique and Myanmar is anticipated in coming weeks.”
Recent updates
Potential partners are invited to submit a proposal for the utilisation of research samples obtained during the FIEBRE study.
Events
Newsletter
Contact us
London School of Hygiene & Tropical Medicine
Keppel Street, WC1E 7HT
Study documents are available from this page, including the study protocol and standard operating procedures (SOPs). For access to other study materials, including data collection tools (case report forms, CRFs), please contact fiebre@lshtm.ac.uk
LSHTM Research acts as an open access repository for FIEBRE publications: https://doi.org/10.17037/PUBS.04652739
You are welcome to contact us with any questions or to request documents that are not yet published.
|
Protocol version |
Description |
|---|---|
|
v1.0 (pdf) |
FIEBRE central protocol version 1.0, 1 Oct 2017 – the study protocol originally approved and implemented at study sites |
| v3.0 (pdf) | FIEBRE central protocol version 3.0, 31 Oct 2018 – supersedes all previous versions |
| v4.0 (pdf) | FIEBRE central protocol version 4.0, 28 Feb 2019 – supersedes all previous versions |
| v4.2 (pdf) | FIEBRE central protocol version 4.2, 15 Apr 2019 – supersedes all previous versions |
| Social science forms | |
| Zimbabwe social science protocol (pdf) | |
| Drug bag questionnaire data collection form (pdf) |
| SOP | Description |
|---|---|
| F.01 (pdf) | Study enrolment: Patient recruitment, screening and enrolment |
| F.02 (pdf) | Informed consent/assent procedures |
| F.03a (pdf) | Completion of clinical CRF for child patients (aged <15 years) on Day 0 |
| F.03b (pdf) | Completion of clinical CRF for adult patients (aged ≥15 years) on Day 0 |
| F.04 (pdf) | Collection of patient samples on Day 0: venous blood, pharyngeal swabs, and urine |
| F.05 (pdf) | Processing of patient samples on Day 0: blood, pharyngeal swabs, and urine |
| F.06a (pdf) | Malaria RDT preparation, reading and results recording |
| F.06b (pdf) | HIV testing and results recording |
| F.06c (pdf) | Processing urinary lipoarabinomannan (LAM) |
| F.06d (pdf) | Cryptococcal antigen testing and results recording |
| F.07a (pdf) | Blood smear preparation and staining |
| F.07b (pdf) | Malaria microscopy: slide reading, recording and internal quality control |
| F.07c (pdf) | Selection of slides for external quality control |
| F.08a (pdf) | Blood culture preparation, interpretation, and results recording |
| F.08b (pdf) | Blood mycobacterial culture preparation, interpretation, and results recording |
| F.08c (pdf) | Urine dipstick use and culture preparation, interpretation, and results recording |
| F.09 (pdf) | Scheduling a patient’s study follow-up and storage of contact information |
| F.10 (pdf) | Completion of CRF for patients on Day 28 |
| F.11 (pdf) | Collection and processing of samples on Day 28: venous blood |
| F.12 (pdf) | Selection, recruitment, and enrolment of community controls |
| F.13 (pdf) | Completion of CRF for controls |
| F.14 (pdf) | Collection and processing of samples from controls: venous blood, pharyngeal swabs |
| F.15a (pdf) | Storage and shipping of dried blood spots from study sites to LSHTM |
| F.15b (pdf) | Sample storage at sites and selection and preparation for shipping participant samples (whole blood, plasma, serum, blood cell pellet, buffy coat, PAXgene tubes and NP/OP swabs) |
| F.15c (pdf) | Selection, preparation, and shipping of bacterial and fungal isolates to the microbiology reference laboratory |
| F. 15d (pdf) |
Preparation, and shipping of mycobacterial isolates to the reference laboratory |
| F.15e (pdf) | Lysis of bacterial and fungal cells for DNA |
| F.15f (pdf) | Shipping FIEBRE samples from LSHTM to reference laboratories |
| F.15g (pdf) | Receiving and storing samples at LSHTM |
| F.16 (pdf) | Identifying and reporting serious adverse events (SAEs) |
| F.19 (pdf) | Collection and storage of PAXgene tube |
| F.20 (pdf) | Processing and storage of FIEBRE samples at LSHTM |
| F.21 (pdf) | Performing the Direct Agglutination Test (DAT) for Leishmania |
| FD.01 (pdf) | Procedure to request updates to eCRF forms |
Publications, research and data produced and contributed to by FIEBRE team members is available including:
- Journal articles
- Conferences, workshops and presentations
- Books, chapters and sections
- Seminars and lectures
- Media
- Blogs
International Journal of Infectious Diseases, July 2022
In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors.
Recent updates
Potential partners are invited to submit a proposal for the utilisation of research samples obtained during the FIEBRE study.
Events
Newsletter
Contact us
London School of Hygiene & Tropical Medicine
Keppel Street, WC1E 7HT
Other research, masters projects and sub-studies will take place in association with the main FIEBRE study, information about these is detailed in this section.
Impact of the COVID-19 pandemic on health care workers and the health care system in Zimbabwe (ICAROZ)
ICAROZ aims to implement comprehensive occupational health services including SAR-CoV-2 testing integrated with screening for major causes of morbidity and mortality in frontline health care workers, with rapid feedback of results to reduce nosocomial spread and trace household contacts.
The occupational health service will be set up in the eight communities and three hospitals (where FIEBRE operating). This will include community health care workers, nurses, midwives, auxiliary nurses, student nurses, cleaners, clerks, security, doctors, radiographers and other staff. The service will include a respiratory symptom screen, temperature, blood pressure and HbA1c measurement as well as an abbreviated physical exam. Anybody with fever and/or respiratory symptoms will be asked to submit a sputum sample for tuberculosis testing (depending on the duration of symptoms) and a nasoparyngeal swab will be taken for SARS-CoV-2. HIV testing either provider-delivered or self-testing using oral mucosal kits will also be offered. Results will be fed back to clients within 24 hours and appropriate measures for self-isolation will be discussed. Contact tracing will be conducted as per the national guidelines.
Lead investigator: Prof Katharina Kranzer
Funder: University of Bristol (Elizabeth Blackwell Institute Global Public Health Research Strand)
Host institute: Biomedical Research & Training Institute
Collaborators: Dr Justen Manasa (Biomedical Research & Training Institute), Prof. Chiratidzo Ndhlovu (University of Zimbabwe), Dr. Hilda Mujuru (University of Zimbabwe), Professor Simbarashe Rusakaniko (University of Zimbabwe)
Setting: hospitals and primary care clinics in/aroundHarare
Population: 6620 community health care workers, hospital and clinic staff
Location: Zimbabwe
Marker of Severity Diagnostics for Evaluating Fever (MOS-DEF)

The Marker of Severity Diagnostics for Evaluating Fever (MOS-DEF) project is a sub-study of FIEBRE funded by Global Good. The objective of MOS-DEF is to develop, evaluate and deploy multiplex assays to measure human blood-borne factors that are released during an immune response. These factors may be informative to indicate the potential causes of fever, and/or, the severity of fever.
Analysis of the assay data will take the form of statistical and computational approaches to identifying individual markers or combinations of markers that could assist in identifying the root cause of fever, the severity of disease in patients presenting with fever and 28 day outcomes of fever cases.
As a sub-study of FIEBRE, the source materials of MOS-DEF are blood plasma collected from inpatient and outpatient settings in the FIEBRE study countries. Analyses will be informed by pre-existing clinical and laboratory data collected during FIEBRE.
Tegwen Marlais is leading the MOS-DEF multiplex assay development at LSHTM.
Markers of immune and endothelial activation
| Biomarker | Name |
|---|---|
| CRP | C-reactive protein |
| PCT | procalcitonin |
| Chitinase | chitinase |
| Ang-1 | angiopoietin-Tie-1 |
| Ang-2 | angiopoietin-Tie-2 |
| Azu/HBP | azurocidin 1/heparin binding protein |
| FLT-1 | fms-like tyrosine kinase-1 |
| sTNFR-1 | soluble tumour necrosis factor receptor -1 |
| TREM-1 | triggering receptor expressed on myeloid cells-1 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| IL-10 | interleukin-10 |
| IP-10 | interferon gamma-induced protein 10 |
| MxA | myxovirus resistance protein A |
Antimicrobial resistance of Gram-negative bacteria from urine specimens (ARGUS)
Antimicrobial resistance (AMR) is a global problem affecting all countries irrespective of income and geographical location, and has been highlighted by the World Health Organization as one of the three most important public health threats of the 21st century. The increase in AMR is driven among others by inappropriate antibiotic use, insufficient or lacking infection control systems and the dissemination of successful bacterial clones harbouring resistance genes. Infections due to drug-resistant organisms are associated with increased mortality and risk of onward transmission, particularly in low-income settings where alternative antibiotics are not readily available, and pose an immense burden on weak health systems.
The ARGUS study aims to investigate the prevalence of and underlying molecular mechanisms for AMR in Gram-negative bacilli causing urinary tract infections in Zimbabwe. Taking into account that inappropriate antibiotic use is a main driver of AMR, this study plans to investigate antibiotic consumption in adults presenting to primary care. This information may be used to interpret the results on prevalence of antibiotic resistance.
The results from this study will be used to inform policy and development of treatment recommendations. Whole genome sequencing results will provide a better understanding of the prevalent resistance genes in Zimbabwe, of the spread of successful clones, and potentially will contribute to developing strategies to tackle AMR.
Lead investigator: Dr Ioana Olaru is an infectious diseases physician and currently undertaking a PhD with LSHTM
Setting: primary care clinics from Harare
Population: 1500 participants with suspected urinary tract infections
Location: Zimbabwe
Supervisors: Katharina Kranzer, Rashida Ferrand, Shunmay Yeung
Carriage of antimicrobial resistance genes in children enrolled in the FIEBRE study
Antibiotic resistance (or antimicrobial resistance - AMR) is a well-recognised threat to global health. Few studies have examined how frequently people in Africa carry bacteria with genes that confer resistance to different antibiotics. The limited data available suggest that the rate of carriage of bacteria with resistant genes is high, even when the antibiotics in question are not widely available locally. There are unanswered questions as to where these genes have come from: previous antibiotic use (either prescribed or over-the-counter); environmentally from the hospital or community, or from eating food where antibiotics have been used in production. We also do not know how often carrying bacteria with AMR genes leads to disease, and whether this leads to a worse outcome for African children.
This study aims to investigate how often children with fever attending inpatient and outpatient facilities in Zimbabwe carry AMR bacteria, whether this relates to the cause of their fever, and leads to worse outcome. The rates of AMR bacterial carriage will be compared before and after admission (for inpatients) and with community controls. In a small number of samples, AMR genes within bacteria will be analysed and compared, to see if the spread of particular genes in hospitals and communities can be mapped, and to explore where the genes may have come from. This study is planned to produce initial data for a wider study in collaboration with vets and geographers looking at the spread of AMR genes in Zimbabwe, and what can be done to prevent that spread.

Project duration: 2017 – 2021
LSHTM lead investigator: Felicity Fitzgerald, UCL Great Ormond Street Institute of Child Health
Co-investigators: Rashida Ferrand, Shunmay Yeung, David Mabey, Ioana Olaru
Funding: Academy of Medical Sciences and the funders of the Starter Grant for Clinical Lecturers scheme
Location: Zimbabwe
Website








