OPT-SMC: Optimising the impact of Seasonal Malaria Chemoprevention - Improving delivery and building capacity for evaluation
Date: May 2020 - April 2024
Location of study: 13 West and Central African countries: Burkina Faso, Cameroon, Chad, Gambia, Ghana, Guinea, Guinea Bissau, Mali, Niger, Nigeria, Senegal, Togo and Benin.
LSHTM Investigators: Susana Scott & Paul Milligan
External collaborators: Prof Dr Jean Louis Abdourahim Ndiaye, Université de Thiès (UT), Senegal; Dr Corinne Merle, UNDP/UNICEF/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR)/ World Health Organization (WHO), Switzerland; Dr Andre-Marie Tchouatieu, MMV Medicines for Malaria Venture, Switzerland
Funding Body: EDCTP, Wellcome Trust, WHO/TDR, MMV
The OPT-SMC project supports national malaria control programmes in West and Central Africa to conduct implementation research on Seasonal Malaria Chemoprevention (SMC) by providing grants and technical assistance. The project facilitates sharing of information and expertise between countries, organizes workshops, and makes key research on SMC published in English available in the French language, with permission from publishers.
SMC programmes delivered SMC to about 22million children in high burden areas in 13 countries in 2019. High coverage is possible thorough door-to -door campaigns, but is not being achieved everywhere. The project therefore supports operational research to understand the local challenges so that delivery approaches can be adapted to reach children each month during the high transmission period. This has included surveys and qualitative studies to understand barriers to uptake in mining communities in Guinea, monitoring of delivery and community attitudes to SMC in northern Ghana, and assessment of the effectiveness of SMC in Benin.
The project has produced video aids in French, English, Hausa, and Portuguese, which can be shared and viewed on smartphones, for use in socially-distanced training and during SMC delivery, to help drug distributors administer SMC safely and effectively. We are evaluating the usefulness of these videos to support SMC delivery during the COVID-19 pandemic.
Evaluating multiple loci modulating susceptibility of African malaria parasites to artemisinin
Date: June 2020 - June 2024
Location of study: Keppel St Laboratories, LSHTM, UK; UHAS Laboratories, Ho, Ghana
LSHTM Investigators: Khalid B. Beshir, Susana Campino, Colin J. Sutherland, Donelly van Schalkwyk
External collaborators: Bismarck Dinko, UHAS, Ghana
Funding Body: UK Medical Research Council (MRC)
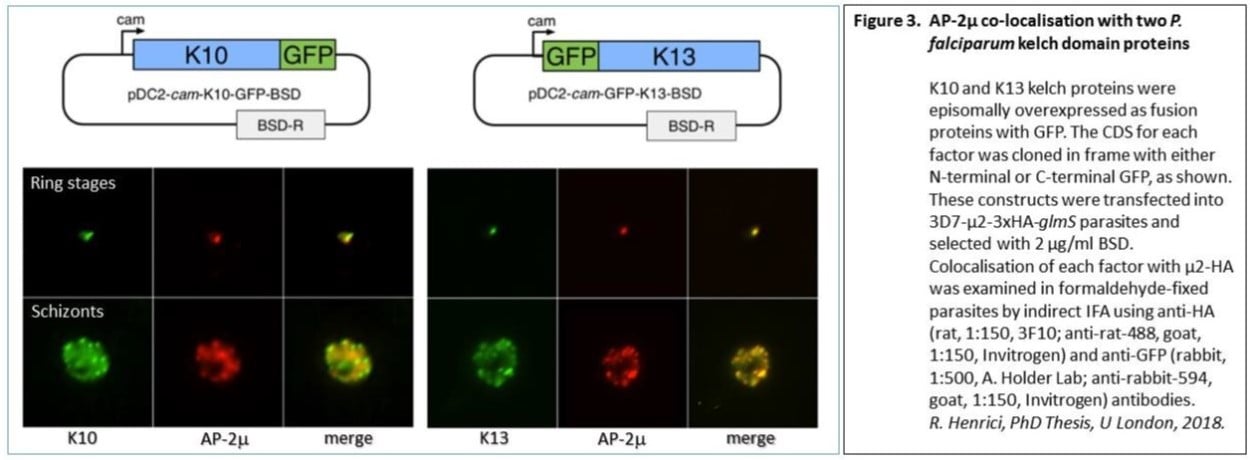
The parasite-killing ability of artemisinin has recently been threatened in the Mekong region. Careful monitoring of malaria drug resistance is therefore needed. Variants of the K13 gene, which encodes a kelch-domain protein, are strongly associated with the loss of effectiveness of artemisinin against these parasites. However, this same phenomenon has not been observed in African malaria parasites - in the small number of cases where drug treatment does not work, K13 mutations are not implicated.
Gene-editing studies have demonstrated a direct effect of 3 other genes in reduced susceptibility to artemisinin : pfap2mu, pfubp1 and pfcoronin. Variants of these genes elicit increased parasite survival in the ring-stage artemisinin survival assay. We found that the trafficking adaptor protein subunit AP-2mu is associated with a compartment in the cell that also contains K13, although we found no evidence of direct interaction between AP-2mu and the K13 protein. There is, however, good evidence of direct interaction of AP-2mu with a different kelch protein , K10.
Our MRC project, awarded in 2020, will support gene-editing studies to characterise new variants in these proteins of interest: pfk13, pfap2mu, pfubp1, pfcoronin and pfk10. We will establish new in vitro cultures from both imported UK malaria cases and from isolates provided by our collaborator Dr Bismarck Dinko in Ho, Ghana, fully test drug susceptibility in each line, and derive whole genome sequences. In gene-editing experiments we will then directly measure the impact of each candidate gene variant on artemisinin susceptibility.
SuNMaP 2 Longitudinal Study
Date: June 2019 - August 2024
Location of study: Kano and Kaduna states, Nigeria
LSHTM Investigators: Dr Bilal Iqbal Avan, Sarah Marks, Dr Joanna Schellenberg, Dr Seyi Soremekun
External collaborators: Dr Zelee Hill (UCL, UK), Dr James Tibenderana (Malaria Consortium, UK), Dr Dawit Getachew, Dr Olusola Oresanya & Dr Chinazo Ujuju (Malaria Consortium, Nigeria)
Funding Body: UK Foreign Commenwealth & Development Office (FCDO)
Support to the National Malaria Programme phase II (SuNMaP 2) in Nigeria is a UK Foreign Commonwealth & Development Office (FCDO) funded programme that aims to strengthen the Nigerian government’s ability to reach the poorest and most vulnerable, with evidence‐based interventions to reduce the malaria burden by adopting a systems-based approach.
LSHTM is leading a four-year mixed method longitudinal study. The primary objective of the longitudinal study is to assess SuNMaP 2’s theory of change to inform the effectiveness of FCDO’s exit strategy from bilateral malaria funding in Nigeria. This will be complemented by ongoing quarterly assessments of SuNMaP 2 to provide programme implementers with information on the degree to which the quality and coverage of malaria control interventions are being implemented; and whether coverage is sustained as partner support to the government is reduced.
Systemic literature review on interventions to improve malaria case management in private medicine retail outlets in sub-Saharan Africa
Date: Feb- Sept 2021
Location of study: sub-Saharan Africa
LSHTM Investigators: Catherine Goodman
External collaborators: Theodoor Visser, Clinton Health Access Initiative
Funding Body: World Health Organisation (WHO)
This systematic literature review aims to identify evidence-based interventions which improved quality of care of malaria with artemisinin-based combination therapies (ACTs) and malaria rapid diagnostic tests in private medicine retail outlets in sub-Saharan Africa. It will serve as basis for in-country programs supported by Global Fund and PMI Impact Malaria, inform strategic frameworks under development, and promote further research based on identified knowledge gaps.
Targeted parasite elimination in the human and mosquito to reduce malaria transmission: A randomised controlled trial
A randomised controlled trial evaluating reactive focal drug administration (rfMDA) versus reactive case detection (RACD), with and without reactive vector control (RAVC) in a low endemic setting of Namibia.
Location of study: Zambezi, Namibia
LSHTM Investigators: Immo Kleinschmidt, Lindsey Wu, Chris Drakeley, Kevin Tetteh
External collaborators: UC San Francisco Malaria Elimination Initiative; University of Namibia; University of the Witwatersrand, South Africa; Ministry of Health and Social Services, Namibia; Zambezi Ministry of Health and Social Services Namibia; UC San Francisco, Division of Experimental Medicine, USA
Funding Body: Bill & Melinda Gates Foundation, Novartis Foundation
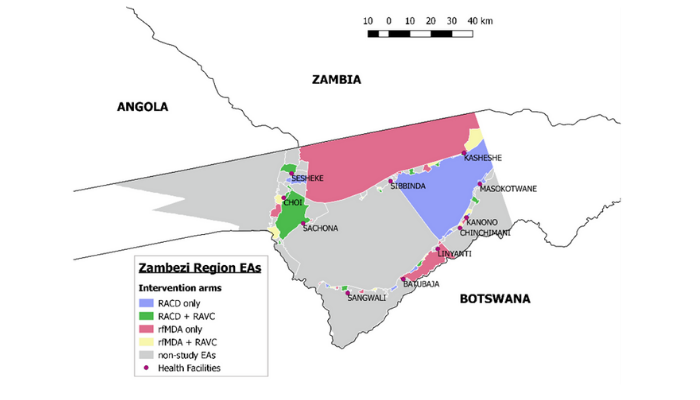
A factorial design cluster-randomised controlled trial was conducted in Zambezi, Namibia to evaluate the effectiveness of reactive focal mass drug administration (rfMDA) and reactive vector control (RAVC) as targeted parasite elimination strategies used alone and in combination. Reactive case detection (RACD) has been widely used as a malaria elimination strategy, but its effectiveness is not well validated due to the limited sensitivity of field diagnostics for asymptomatic infections. This study aimed to test the effectiveness of alternative elimination strategies targeting the parasite in both humans and mosquitoes, using a factorial design cluster randomised controlled trial.
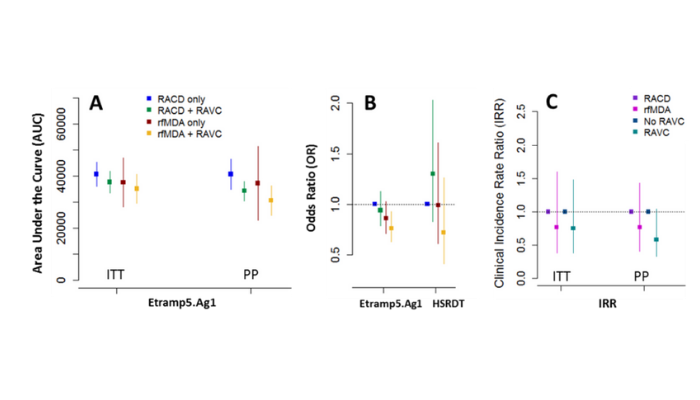
Between January and November 2017, 56 enumeration areas in Zambezi, Namibia were randomised to four study arms: reactive case detection only (RACD), reactive case detection with reactive vector control (RACD + RAVC), reactive focal mass drug administration only (rfMDA), or reactive focal mass drug administration with reactive vector control (rfMDA + RAVC). Factorial trial design enabled comparisons of test vs. control interventions targeting the human reservoir (rfMDA vs. RACD), the mosquito reservoir (RAVC vs. no RAVC) and in combination (rfMDA + RAVC vs. RACD only). The largest effect was observed in the combined intervention arm. Locally-acquired cumulative incidence in the rfMDA + RAVC intervention arm was 18.5 per 1000 individuals compared to 30.8 cases per 1000 in the reactive case detection control arm.
The adjusted hazard ratio in the combined intervention compared to control was 0.56 (0.36 – 0.87, p=0.01). A cross-sectional survey was conducted at the end of the malaria season (May – August 2017) to measure P. falcparum infection prevalence using two rapid diagnostic tests (CareStart Malaria HRP2/pLDH(Pf/PAN) and ultra-sensitive HRP2-based Alere™ Malaria Ag P.f RDT)) quantitative PCR, and a panel of over 20 serological markers on the Luminex quantitative suspension array (qSAT) immuno-assay. The serological analysis led by LSHTM researchers observed that several markers of recent malaria exposure, such as Etramp5.Ag1, were correlated with primary trial endpoints (clinical incidence rate ratio) and may have potential as secondary endpoints in future cluster randomised trials.
Diagnostic performance of ultra-sensitive rapid diagnostic tests for detecting asymptomatic P. falciparum on the Cambodian-Thai border
Location of study: Oddor Meachey Province, Cambodia
LSHTM Investigators: Shunmay Yeung, Nicola James, David McGregor
External collaborators: Soy Ty Kheang (Health and Social Development); Po Ly, Siv Sovannaroth (National Center for Parasitology, Entomology & Malaria Control, Cambodia); Saorin Kim, Nimol Khim, Benoit Witkowski (Institut Pasteur du Cambodge, Cambodia)
Funding Body: UK DFID through the Tracking Resistance to Artemisinins Collaboration
Proposed interventions for eliminating drug resistant P. falciparum malaria, include the targeting of asymptomatic carriers through screening and treatment. We compared the diagnostic performance of the recently developed ultra-sensitive rapid diagnostic test (uRDT) under field conditions in Cambodia, compared to screening with conventional RDTs (cRDT) and polymerase chain reaction (PCR). This study was nested within the PACES study, a cluster randomised control trial of active case detection for malaria which was carried out in Oddar Meanchey Province in Northwest of Cambodia.
We carried out a comparison of uRDTs with conventional RDTs and PCR under two field conditions: active case detection and a cross-sectional survey. In total 2,729 tests were carried using all three diagnostic tests; 678 during active case detection and 2,051 during the cross-sectional survey. The positivity rate for P. falciparum infections by qPCR analysis was 3.8% (26/678) and 0.48% (10/2051) for active case detection and cross-sectional survey respectively. In both these contexts uRDT were shown to have similar sensitivity and specificity to cRDT. The results of this study therefore do not suggest an improved performance of uRDTs over conventional RDTs when used for either active screening or cross-sectional surveys in very low prevalence areas such as Northern Cambodia.
Proactive case detection and community participation for the elimination of drugresistant malaria in Cambodia Study (PACES)
Location of study: Oddor Meachey Province, Cambodia
LSHTM Investigators: Shunmay Yeung, Nicola James
External collaborators: Soy Ty Kheang, Somphos Chhloeung, (Health and Social Development); Po Ly, Hu Rekol, Siv Sovannaroth, (National Center for Parasitology, Entomology & Malaria Control, Cambodia); Benoit Witkowski, Didier Ménard (Institut Pasteur du Cambodge, Cambodia); Koen Peeters (Institute of Tropical Medicine, Belgium); Iveth Gonzalez and Xavier Ding (Foundation for Innovative New Diagnostics, Switzerland)
Funding Body: UK DFID through the Tracking Resistance to Artemisinins Collaboration
The spread of artemisinin resistant P. falciparum malaria is one of the biggest threats to global malaria control and elimination. Cambodia, which is at the epicentre of multidrug resistant malaria, declared a goal of eliminating malaria by 2025. However it is unclear how to achieve this operationally, for example who to target for strategies such as active case detection, what screening tests to use and how acceptable these interventions are to the affected communities? The aim of our study was to address some of these questions and to contribute to the national and regional efforts to eliminate drug resistant malaria.
We carried out a mixed methods study at the core of which was a cluster randomised control trial of active case detection using village malaria workers in 130 villages in Oddor Meanchey province. We confirmed how focal and dynamic malaria is in this study area, and the importance of working very closely with the community. In most villages there was no local transmission, and little point in screening people who had not recently been to the forest. For at-risk contacts of patients who had presented to the village malaria worker with symptomatic malaria, around one-fifth tested positive for malaria.
The intervention was generally very well accepted however the majority of the asymptomatic malaria that we uncovered was not due to P. falciparum but P. vivax and that the relapsing nature of P. vivax poses as much, if not more of a burden in affected communities. However, this is not related to drug resistance. Future plans include operational research focusing on providing radical cure for P. vivax with prior G6PD testing. By understanding more about malaria and the at-risk population in these areas and sharing the lessons we have learnt in implementing active case detection, we hope to contribute to the national and regional efforts to eliminate drug resistant malaria.
Non-inferiority of targeted reactive IRS compared with generalised routine IRS and determination of transmission hotspots
Location of study: South Africa
LSHTM Investigators: Immo Kleinschmidt, Jackie Cook, David Bath, Catherine Pitt, Joseph Biggs
External collaborators: Maureen Coetzee (Wits Research Institute for Malaria, University of the Witwatersrand, South Africa); Natashia Morris, Rajendra Maharaj (South African Medical Research Council, South Africa); Aaron Mabuza (Mpumalanga Provincial Malaria Control Programme, South Africa)
Funding Body: MRC/DFID/Wellcome Trust Joint Global Health Trials
In areas of very low, pre-elimination malaria transmission the low number of incident cases may call into question the resources needed for spraying all houses annually with insecticide (generalised indoor residual spraying (GIRS)). An alternative that may be more cost-effective is to target IRS, reactively spraying only houses in the immediate neighbourhood of incident cases as they arise.
62 clusters consisting of settlements of approximately 5000 persons each in the provinces of Mpumalanga and Limpopo in South Africa, were randomly allocated to receive the currently practised GIRS whilst the other half received reactive targeted IRS (TIRS) in the immediate neighbourhood (about 8 houses) of every passively reported local case. Non-inferiority of TIRS was assessed as passive incidence of no more than 1 case/1000 per year above that of the GIRS arm. Dried blood spots were collected from neighbourhoods of index cases, and in randomly selected neighbourhoods where there were no cases occurred, to determine, using serology, whether new cases arise predominantly in areas of previous exposure to parasites.
Incidence in the targeted IRS was higher than in the generalised IRS arm by 0.29 cases per 1000 (95% CI -0.53-1.10). Antibody prevalence showed that cases arise in neighbourhoods with raised levels of previous exposure to malaria parasites. Although evidence of non-inferiority of TIRS compared to GIRS was weak, TIRS appears to be a safe, more sustainable method of deploying IRS in areas of very low transmission. Since the neighbourhoods of passively detected cases are characterised by raised levels of past exposure to parasites, it is important that these areas are targeted with additional interventions such as focal IRS.
Freedom from Infection: confirming the interruption of malaria transmission for elimination
Location of study: Cabo Verde, Indonesia, Haiti, Laos, Thailand, Vietnam
LSHTM Investigators: Lindsey Wu, Henry Surendra, Gillian Stresman, Chris Drakeley
External collaborators: Kim Linblade and Abdisalan Noor (WHO Global Malaria Programme), Arnaud Le Menach and Justin Cohen (Clinton Health Access Initiative), Pak Yono Supargiyono (Universitas Gadjah Mada, Indonesia), Adam Bennett and Andrew Lover (UC San Francisco Malaria Elimination Initiative), Adilson Jose de Pina (Cabo Verde Ministry of Health)
Funding Body: Bill & Melinda Gates Foundation
While a number of countries are targeting malaria elimination, epidemiologically confirming the absence of cases or infections is challenging. This project is adapting “Freedom from Infection” surveillance tools, used extensively in veterinary epidemiology, to guide national malaria control programmes in the design of optimal elimination surveillance strategies.
Freedom from Infection (FFI) surveillance tools have been used in veterinary epidemiology to demonstrate that infection in a population is below a defined threshold. These approaches characterise the absence of transmission within a degree of statistical certainty. This project is adapting FFI tools specifically for malaria in collaboration with national malaria control programmes (NMCPs) and research groups across a range of countries currently targeting elimination. Our analysis estimates the probability that regions are free from malaria transmission using routine health facility data or community surveys.
Analysis also includes determining feasible time frames required to confirm elimination at national or subnational levels and quantifying the value-added of additional diagnostics or surveillance endpoints to more accurately estimate freedom from infection. Preliminary analysis, such as from the Kulon Progo Regency in central Java, Indonesia, has shown that monthly surveillance data over the course of one year can be used to estimate the probability of freedom from malaria infection at the health facility level. Work is currently ongoing to demonstrate the use of these tools with surveillance data at national and subnational levels in Laos, Thailand, Vietnam, Haiti, and Cabo Verde. We are also partnering with NMCPs and the WHO to determine how these tools can be designed to contribute to programmatic decision-making for elimination certification.
Can dogs identify people with malaria parasites? A proof-of-principle study
Location of study: United Kingdom, The Gambia
LSHTM Investigators: James Logan, Sarah Dewhirst, Chelci Squires
External collaborators: Steven Lindsay (Durham University, UK); Umberto D’Alessandro & Margaret Pinder (MRC Unit The Gambia at the LSHTM); B. Kandeh (National Malaria Control Programme, Banjul, The Gambia); Jenny Corish, Clare Guest & Mark Doggett, (Medical Detection Dogs, UK); S. Morant, (Medicines Monitoring Unit, University of Dundee, UK)
Funding Body: Bill & Melinda Gates Foundation
The task of malaria elimination would be simpler if a noninvasive method was available for detecting infected individuals in populations where the number of malaria cases is low; infected individuals could then be treated with antimalarials. Dogs have a highly developed sense of smell and may be able to detect volatiles released from people carrying malaria parasites. We carried out a proof-of-principle study to determine whether trained dogs could detect malaria infections in Gambian children aged 5-13 years old.
Why was this study done?
- The World Health Organization has a vision of a malaria-free world. Since 2000 *eight countries have been certified as free from malaria, with a further 12 reporting no indigenous cases. Once a country or region has been declared malaria-free detecting individuals with asymptomatic infections is critical so they can be treated with antimalarials and the transmission of the disease prevented.
- Ideally a non-invasive diagnostic device that can detect malaria-infected subjects would be an advance, helping to keep the disease out of malaria-free countries.
- In The Gambia, West Africa, the number of malaria infections has declined substantially since 2000 and provides an ideal site for detecting malaria at low frequencies.
- We tested the hypothesis that dogs, with their powerful sense of smell, could be trained to distinguish between individuals with and without Plasmodium falciparum malaria
What did the researchers do and find?
- Gambian schoolchildren were screened to detect malaria parasites in their blood to determine those carrying parasites and those without.
- We provided socks for the schoolchildren to wear overnight in order to collect samples of their foot odours.
- Two dogs were trained to distinguish between sock samples from malaria-infected and uninfected individuals tested in a double blind fashion.
- Two dogs were able to identify strips of socks from malaria-infected and uninfected individuals with a sensitivity of 70% and 73% and a specificity of 90% and 91%.
What do these findings mean?
- Dogs can identify malaria-infected individuals by their odour with a credible degree of accuracy. • In the future artificial odour sensors may be able to detect malaria parasites.
- Until then, trained dogs may be useful at ports of entry to detect asymptomatic malaria carriers entering malaria-free countries.
- Further studies are needed to detect falciparum malaria in people from different parts of the world before medical detection dogs can be used in the field.