One bacterium, disguised in multiple forms, was responsible for an estimated 18% of all childhood deaths worldwide in 2005.
This translates to almost one million deaths caused by this one agent, and several million more people experiencing the wide range of symptoms and diseases the bacteria can cause.
Children in developing countries were affected the most, with severe cases up to 10 times higher in African countries. But the bacteria had, and continues to have, a truly global burden.
The offender’s name: streptococcus pneumoniae.
Infection with this pathogen through its multiple identities can cause conditions as mild as sinusitis (inflamed sinuses) and middle ear infections, but it can also cause significantly more severe diseases, including pneumonia, meningitis and septicaemia.
These all combine to be known collectively as pneumococcal disease, with children under the age of two most at risk.
More than 90 serotypes – distinct versions — exist for this particular bacterium, though only a handful cause disease, leading global health experts to have spent the past few decades mastering the one weapon they hoped could kill them all: a vaccine.
“Vaccines have been one of mankind’s greatest inventions,” said Professor Brendan Wren, Dean of the Faculty of Infectious and Tropical Disease at the London School of Hygiene & Tropical Medicine. “[They] have proven to be very useful against strep pneumonia.”
The first vaccine was licensed for use in 2000, after which a trial was conducted by the School and other partners in The Gambia to test its effectiveness. After promising results published in 2005, its introduction became more widespread globally and infection numbers soon fell dramatically.
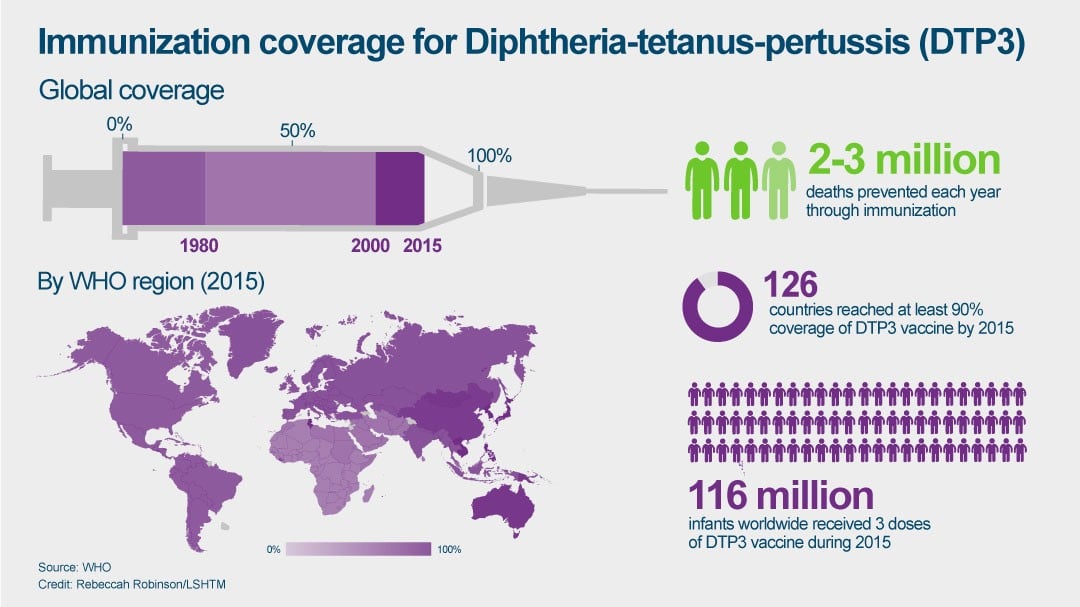
By 2015, having gone through various iterations, the pneumococcal vaccine had been introduced in 129 countries, and global coverage was estimated to be 37% among children under the age of two.
The challenge, however, has been creating a vaccine that protects against all the harmful strains of streptococcus pneumoniae, as well as one that is affordable to all countries in need.
The first version protected against seven of the strains, then 10, and now some countries are using a form protective against 13 strains, but more still need to be targeted – as many as 76 remaining serotypes. The effect of vaccination is, of course, positive overall. But the increase in non-vaccine types tempers the overall effect of vaccination.
The current method of production also means each dose can cost as much as $50, making it an expensive vaccine.
“More than a million people worldwide still die from the disease (each year) despite the fact that there’s a vaccine,” said Prof Wren. “There’s a real incentive to produce these at lower cost and to be more efficacious and cover as many strains as possible.”
Prof Wren’s team has developed a game-changing technology using E.coli that they believe could create the types of vaccines used to fight pneumococcal disease. Known as a glycoconjugate vaccine, it protects against a wider range of serotypes and, perhaps more importantly, costs just US$1 per vaccine.
“Our new vaccine technology can make these in E.coli very cheaply, so you have an inexhaustible and renewable supply of pure material,” said Prof Wren.
This platform also goes beyond pneumococcal disease, and could be used to create vaccines against multiple bacterial diseases.
A (cheaper) glycoconjugate future
Glycoconjugate vaccines are a design of vaccine currently used to fight three types of bacteria: Haemophilus influenza, which causes meningitis, pneumonia and cellulitis to name a few, neisseria meningitidis, and streptococcus pneumoniae.
The vaccines work by combining a polysaccharide, also known as a glycan, from the surface of the bacteria being targeted with a protein that helps stimulate a T-cell immune response in the human body. This elicits an immune reaction against the particular bacteria, ready to attack if it were to ever invade the body.
“Glycoconjugate vaccines have been shown to be very effective, because you get long memory – T-cell dependent and T-cell independent response,” said Prof Wren. “This means that they’re very effective and can be used in children as well.”
Though this new method is currently only used to produce three vaccines, these glycoconjugate vaccines have been proven to be very effective, according to Prof Wren. “More than a billion doses have been used in humans worldwide,” he said.
The current production process, however, — not Prof Wren’s new, pilot, method — involves purifying the outer capsule of a bacterium and then growing the intended glycan, or polysaccharide, in the lab. The protein needed for the mixture is also grown in parallel for both components before it’s chemically synthesized to join them together.
“This is a multi-step procedure and is very expensive commercially to produce,” said Prof Wren.
His new platform uses E.coli to conduct all these steps in one place by inserting both components into the E.coli along with an enzyme that binds them together.
“The enzyme couples the sugar to the protein and we were able to put this in E.coli to produce recombinant glycoproteins in E.coli,” he said. “We can almost now pick any protein and put it with any glycan, meaning we can have multiple combinations of proteins and we can go beyond the current glycoconjugate vaccines that are available.”
This extended range applies to diseases beyond the current three vaccines using this approach. Prof Wren is now using his transformed E.coli to develop vaccine candidates against three lesser known, but fatal, diseases: tularemia, affecting people in the US, Q fever, found worldwide (it has affected troops in Afghanistan), and melioidosis, mostly affecting people in Thailand and Northern Australia.
“There are no effective vaccines against these, so there is a need,” he said, adding, “We still don’t have vaccines against all organisms, particularly against some of the most dangerous pathogens.”
Prof Wren’s platform is a bit too efficient, he admits, as candidates can be developed so quickly that the ability to actually test them can’t keep up. Field trials require time and resources.
But he hopes his methodology will at least soon lead to the fruition of more effective pneumococcal vaccines as while the current vaccine has shown stark reductions in numbers infected – as much as 55% in trials in The Gambia – they remain expensive and less potent than they could be.
“If you look at the pneumococcal conjugate vaccine, where the vaccine has been rolled out there is an absolutely clear reduction of the incidence of pneumonia, hospitalisation of pneumonia and deaths from pneumonia,” said Dr Martin Friede, Coordinator of the Initiative for Vaccine Research at the World Health Organization.
“Along with other studies and public health efforts we did contribute quite a lot to restoring public faith in the vaccine.”
Professor Liam Smeeth, London School of Hygiene & Tropical Medicine
Dr Friede highlighted that although the vaccine targets children, older populations have also benefited from the lack of circulating infection. “For old people, pneumonia can be a very severe fatal infection and so by immunising the children, we are preventing the spread of pneumonia and the infection,” he said.
The war on this disease is far from over, as some strains of the bacteria are now rendered powerless, the remaining ones have stepped up to the plate.
“We continue to do a lot of work on pneumococcal vaccination,” said Professor John Edmunds, Dean of the Faculty of Epidemiology and Public Health at the School. “The vaccine has limited coverage … and by vaccinating against those 13 types do we get an increase in the other types? Yes we do, so what’s the impact of that?”
Because streptococcus pneumoniae has as many as 90 serotypes, protecting people against 13 of them has led to the remaining types flourishing and having more impact on the population.
This is something Prof Edmunds has shown through his mathematical modelling work at the School, using data to predict outcomes of various scenarios, such as the spread, or potential spread, of pneumococcal infection.
“We can use mathematical models to see ‘what if we did this’ or ‘what if we introduced a vaccination programme like this’ and ‘what impact might that have’ and ‘should we have a campaign’ and ‘what age group, how many doses?’” he explained.
As a result, his work is crucial in deciding how, once proven effective, or once licensed, a vaccine should be implemented in the real world – and where it should be introduced, if at all.
“I work closely with decision-making bodies in the UK and internationally to help decide what vaccines we should introduce,” said Prof Edmunds.
Once something is then implemented, further data, and the models resulting from that, can monitor how effective the new program is and what could be changed to improve it. “Is the epidemiology evolving in the way we thought, or do we need to make changes or add another dose, or even reduce doses?,” he asks.
Dr Friede agrees that modelling is a crucial part of the entire vaccine development process. “If we don’t model, it’s chaos,” he said. “If we don’t model we don’t really know how limited resources should best be used … and if we don’t have the models, we are walking blindly – maybe the wrong population, not immunising the right people.”
One prime example is the control of seasonal influenza, where new strains of the virus arrive each year and circulate among populations during the winter months.
Vaccines exist to fight the infection, but are not fully protective and are generally targeted at key groups within the population to more effectively — and cost-effectively — control its spread.
“This is a vaccine where we need to adapt the vaccine every single year to the circulating strain. It is at best moderately efficacious,” said Dr Friede.
In recent years, a new subset of the population came to light and those modelling the disease solved a new mystery.
Learning from the ever-evolving influenza
“We’ve been looking at what’s the best way to control flu,” said Prof Edmunds.
At first, the seasonal flu vaccine was targeted to high-risk groups, such as people with heart disease and asthma, but was soon extended to include people over the age of 65.
“It’s an obvious group to vaccinate,” said Prof Edmunds. “Those are the people who are most likely to suffer severe consequences.”
But his team soon realised that this was not necessarily leading to a reduction in the spread of influenza in the population. Though the elderly were the ones most likely to suffer extensively from an infection, they weren’t the ones spreading it within the community.
These “spreaders” came in a much smaller, younger form: children.
“When we started analysing them, it was pretty clear that children play a really key role in the spread of flu and children have a very high incidence of flu,” said Prof Edmunds.
Through models, his team saw that when looking at the incidence of infections from one year to the next, children were the most likely to be infected and carry quite a high burden.
The years of focusing on the elderly had avoided deaths and severe infections, but not reduced spread by very much. “It became pretty clear that vaccinating the elderly and risk groups is not the best way to control flu, because they’re not the key spreaders,” said Prof Edmunds.
This insight and the evidence, through models, backing up this theory led to Prof Edmunds, using his seat on the influenza subgroup of the Joint Committee on Vaccination and Immunisation in the UK, to make recommendations to extend the seasonal influenza vaccine to young children.
A phased introduction of this began in the UK in 2013, and by the 2016/17 flu season guidance was to provide the vaccine to most child age groups as high as primary school ages.
“If you vaccinate children, then it has a direct benefit for the children, who have a high risk of getting flu, but it also reduces the amount of flu circulating in the community, so it’s good for everybody,” said Prof Edmunds.
His team have modelled an extensive range of vaccines over the years, including ones proven to be effective against Ebola towards the end of the 2014 epidemic in West Africa and the somewhat controversial introduction of a vaccine against Human Papillomavirus (HPV) in girls aged 12-13.
The HPV vaccine is given to prevent later infection with this sexually transmitted virus, which in turn can cause cervical cancer. The sexual nature of the infection led to some push-back by parents.
“It was this idea that you vaccinate your daughters against a virus that’s sexually transmitted in hope she won’t get infected, in hope she won’t be at risk long term of cervical cancer,” said Professor Liam Smeeth, Head of the Non-Communicable Disease Epidemiology Unit at the School.
“It was a chain of confidence needed that perhaps some people didn’t have,” he said.
However, the vaccine has, generally, had good uptake and reception among the population, according to Prof Edmunds.
“[These concerns] don’t seem to have had much impact on HPV vaccine coverage,” he said, adding the benefit that, “if you vaccinate the women, you will also protect the heterosexual men, they can’t get it from anywhere else.”
The public response to the new influenza recommendation made by Prof Edmunds also showed some concern by parents. Some questioned one more vaccine being added to their child’s list that wasn’t fully protective anyway and one that is mainly needed to protect the wider community, rather than their child.
This greater good in terms of protecting the community, however, is part of the general rationale of vaccines – to provide a population-based shield to block a virus, bacteria, or parasite, from continuing its life cycle.
Take for example the measles vaccine.
As one of the most effective vaccines known, providing as much as 97% protection against measles infection, the removal of measles from an entire population needs at least 90% of people to be vaccinated so that the virus has nowhere to spread, or replicate. This is known as the herd immunity threshold.
But a single scientific paper in The Lancet in 1998, in which British scientist Andrew Wakefield, claimed there to be a link between people receiving the Measles, Mumps and Rubella (MMR) vaccine and developing autism, led to people questioning use of the vaccine and a resulting drop in the numbers of children receiving it.
Keeping the public on side
“A public concern was prompted by this paper,” said Prof Smeeth whose research to restore vaccine confidence began in response to the paper “We’d seen coverage of the vaccine dropping very sharply, to levels that would lead to measles outbreaks.”
Outbreaks of measles were seen in the borough of Lambeth in London and in smaller communities in Wales, as pockets of people grew with no protection to the virus.
“Vaccine coverage dropped and we had a generation of children coming through, a moderate proportion of which had not been vaccinated so measles could take hold,” said Prof Smeeth.
In 2004, fewer than 82% of children were being given MMR, making it lower than the level needed to avoid an epidemic.
This startling data ignited Prof Smeeth’s passion for maintaining public faith and he set out to uncover the truth and, in turn, prove the value of people giving their children the MMR jab.
By analysing anonymised medical records of children across hundreds of medical practices in England and Wales, his team researched whether children with autism, or other pervasive development disorders, were more likely to have received the MMR vaccine. The vaccination history of almost 1,300 children diagnosed with these disorders between 1987 and 2001 were compared against more than 4,400 control children with no record of autism.
The results showed no association between autism, or related disorders, and children receiving the vaccine. “We showed pretty convincingly that there was no link with autism,” said Prof Smeeth.
Wakefield was struck off the medical register soon after his paper, for fabricating research, but unfortunately continues to appear in the public sphere insisting validity in his findings. He recently directed an anti-vaccine film, “Vaxxed.”
“There are groups that are very active in trying to question the safety of vaccines, unfortunately some of these people are now getting very high visibility,” said Friede, who is worried about the future of such people gaining public support. “There does appear to be a tendency at the moment that opinion has as much value as scientific evidence … that may have some negative outcomes for the health of the population.”
Prof Smeeth believes that transparency of all information and findings with the public is key to maintaining their support for immunisation.
“Along with other studies and public health efforts we did contribute quite a lot to restoring public faith in the vaccine,” he said. “When we published our study … vaccine coverage started to climb again.”
Almost 20 years after the scare, Prof Smeeth believes confidence in the vaccine has mostly been restored, but added that “there will always be slight uncertainties and people on each end the spectrum: sceptics to people with legitimate concerns, through to enthusiasts.”
MMR is one of many routine vaccinations, in addition to those against diphtheria, tetanus and pertussis (DTP3), given to newborns and infants worldwide to shield them from diseases that were once prevalent and fatal, but are now rare and near elimination.
Ensuring the rarity of these diseases continues requires public confidence – and understanding.
“I think what’s happened in many countries now is that deaths from infectious disease have become so rare that people have forgotten it’s a reality,” said Dr Friede. “The moment an adverse event happens it becomes very visible … and there are groups that are very active in trying to question the safety of vaccines.”
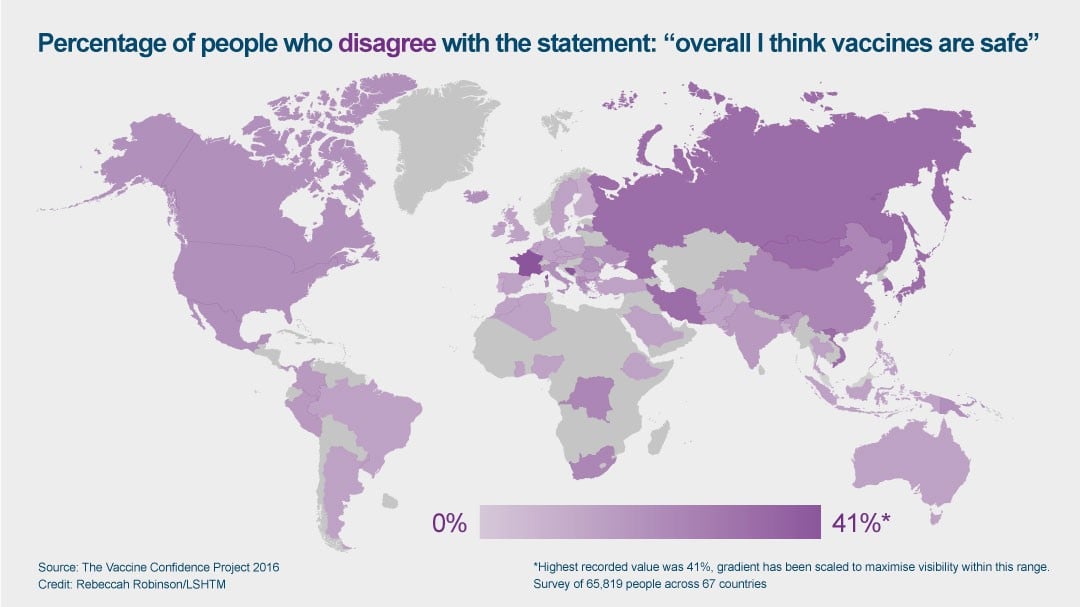
Confidence in the idea of vaccines varies significantly in different regions of the world, as shown in a recent study led by the School’s Vaccine Confidence Project. 65,819 people were surveyed across 67 countries, and the researchers found that and seven of the 10 least confident countries were in Europe.
More than 40% of respondents in France and 36% of respondents in Bosnia & Herzegovina reported that they disagree that vaccines are safe.
The global average, however, was just 12%.
The research, led by Dr Heidi Larson from the School highlighted the need to keep track of attitudes regularly in order to, “quickly identify countries or groups with declining confidence … and then act swiftly to investigate what is driving the shift in attitudes,” she said.
For some groups, any resistance to immunisation may not be fear of their harms, or side-effects, but instead more practical measures — like family size. This is a factor affecting the Charidi (Orthodox Jewish) community in London.
“There are some challenges, with GPs (general practitioners) not having the correct facilities,” said Rabbi Avrhom Pinter, Chairman of the Charidi Health Forum. Rabbi Pinter added that with many children to look after and take out with them, women often need larger, accommodating spaces which are often not the case in the small medical practices of North London.
Mothers are left concerned about where to breastfeed, a practice the women of this community are not comfortable doing in public.
“Most GPS don’t have feeding areas … have long waiting times, it’s practical reasons that can be overcome,” said Rabbi Pinter. But through his work to highlight the needs of his community, he announced that the Hackney GP confederation will be spending £2,200 over the next two years to address this issue, one approach being the introduction of mobile health units. “I’m confident that we will get there.”
The unpredictability of humanity
Human attitudes, and behaviour, are something Prof Edmunds explained are more complicated to model. Teams do not have good models of these, making it harder to predict and factor in when deciding who to target with certain vaccines and when.
“These issues crop up on a fairly regular basis,” he said. “We can’t really predict that element of it as we don’t really have data to use with that.”
“I cannot predict which of these (scares) will get traction and cause a decline in coverage and how much of a decline and so on,” he added. “That is so unpredictable, we don’t have any way of modelling that.”
The only hope, therefore, is to monitor changes regularly, and any change in vaccine uptake needs to be addressed as it can have an impact on a public health level.
“There are people who have a range of beliefs around vaccines and some who reject the science,” said Prof Smeeth. “I’m a clinician and a scientist so I would disagree and try and convince them I am right, but some people are going to be attached to their beliefs and that’s something we have to face.”
Title and cover image:© WHO/S Hawkey.